CARBON MONOXIDE IN WORKPLACE ATMOSPHERES
| Method Number: | ID-210 |
| Matrix: | Air |
| OSHA Permissible Exposure Limits Final Rule Limits: |
35 ppm Time Weighted Average (TWA) 200 ppm Ceiling (5-min sample) |
| Transitional Limit: | 50 ppm TWA |
| Collection Procedure: | Each sample is collected by drawing a known volume of
air into a |
| Recommended Air Volume: | 2 to 5 liters |
| Recommended Sampling Rates TWA Determination: Ceiling Determination: |
0.01 to 0.05 L/min 1 L/min |
| Analytical Procedure: | A portion of the gas sample is introduced into a gas sampling loop, injected into a gas chromatograph, and analyzed using a discharge ionization detector. |
| Detection Limits (TWA, Ceiling) Qualitative: Quantitative: |
0.12 ppm 0.40 ppm |
| Precision and Accuracy Validation Range: CVT(pooled): Bias: Overall Error: |
17.2 to 63.6 ppm 0.025 +0.058 ±10.8% |
| Special Requirements: | Samples should be sent to the laboratory as soon as possible and analyzed within two weeks after collection. |
| Method Classification: | Validated method |
| Chemist: | Robert G. Adler |
| Date: | March, 1991 |
Commercial manufacturers and products mentioned in this method are for descriptive use only and do not constitute endorsements by
USDOL-OSHA. Similar products from other sources can be substituted.
Inorganic Methods Evaluation
Branch
OSHA Salt Lake Technical Center
Salt Lake City, Utah
1. Introduction
- The upper analytical range used during the evaluation of this method was about 430 ppm; the upper linear range for CO may be much larger than this concentration.
- The qualitative detection limit was 0.12 ppm for a
1-mL gas sample (size of GC gas sampling loop). The quantitative detection limit was 0.40 ppm. If necessary, a larger sampling loop can be used to achieve a lower limit of detection. - The sensitivity of the analytical method [using analytical
conditions stated for a Tracor 540 GC (Tracor Instruments Austin,
Inc., Austin TX) and
Hewlett-Packard 3357 Laboratory Automation System, Revision 2540(Hewlett-Packard Co., Avondale PA)] was taken from the slope of the linear working range curve (1.70 to 63.6 ppm range). The sensitivity is 1,970 area units per 1 ppm. (For the HP 3357 Automation System, 1 area unit = 1 µV· s.) - The pooled coefficient of variation for the sampling and analytical method from 17.2 to 63.6 ppm was 0.025.
- The average recovery of generated samples taken in the
17.2-63.6 ppm range at 50% RH was 105.8%. The range of bias was -0.01 to +0.10. The Overall Error (OE) was ±10.8%. - Precision and accuracy data were derived from generated samples and prepared standards that were aged 4 days or less. The stability of CO in sampling bags is acceptable up to 2 weeks after sample collection.
- Stability tests indicated that significant scatter in the results and lower recoveries tended to appear after prolonged storage. Use of new bags free of small leaks and internal deposits may prolong sample stability. Samples should be analyzed as soon as possible to minimize storage problems.
1.1 History
The recent change in the TWA Permissible Exposure Limit (PEL) for
carbon monoxide (CO) from 50 to 35 ppm (5.1.) and the inclusion of a
Ceiling of 200 ppm
Previous classical methods found in the literature for the analysis of CO have consisted of the collection of air samples in gas bags or canisters with analysis either by infrared absorption spectrophotometry (5.6.), electrochemical means (5.7.), or gas chromatography using a flame ionization detector (5.8.).
Gas chromatography (GC) offers many advantages for CO analysis (5.4., 5.9.); however, because the sensitivity for CO by a flame ionization detector (FID) is extremely low, it is necessary to react hydrogen with CO on a catalyst such as heated nickel to produce methane before FID analysis can be performed at the levels of interest (5.5., 5.8.). This methanization procedure introduces an additional step, since it is necessary to identify any methane in the sample, and makes the analysis more complex. Also, the hydrogen gas used in the conversion of CO to methane is sometimes contaminated with methane.
With the recent development of the discharge ionization detector
(DID) for use with GC analysis, it is possible to measure CO
concentrations directly at very low levels (5.10.). Helium is
generally used as the sample carrier gas and as the ionized species.
In the detector, helium is passed through a chamber where a glow
discharge is generated and
1.2. Principle
1.2.1. A low-flow rate sampling pump is used to capture a known
volume of air in a
1.2.2. A GC fitted with a gas sampling loop and a DID is used to assess CO sample concentrations.
1.3. Method Performance
1.3.1. Range, detection limit, and sensitivity:
1.3.2. Precision, accuracy, and stability:
1.4. Advantages and Disadvantages
1.4.1. The method is specific for CO. The method is also applicable in measuring compliance to Indoor Air Quality Standards for CO [9 ppm (8 h), 35 ppm (1 h)] (5.11.).
1.4.2. Using similar procedures, sampling and analysis for carbon dioxide (CO2) is also possible provided the molecular sieve column is eliminated from the gas stream during CO2 analysis.
1.4.3. Gas sampling bags are employed and may be somewhat inconvenient to use.
1.4.4. Changes in humidity do not affect sample collection.
1.4.5. The bulk of the sample is not destroyed during analysis. Other potentially toxic gases may also be analyzed from the same sample.
1.4.6. The gas bags used as sample collection media are reusable.
1.4.7. The method requires the use of a GC equipped with a DID.
1.4.8. Analytical time required per sample is within 20 min when using the conditions specified.
1.4.9. Gas bag samples are stable for approximately 2 weeks. Samples should be analyzed as soon as possible.
1.5. Physical Properties of CO (5.12., 5.13.)
| Molecular weight | 28.01 |
| Molecular formula | CO |
| Appearance | Colorless, odorless gas |
| Explosive limits in air | 12.5 to 74.2% (v/v) |
| Autoignition temperature | 651 °C |
| Melting point | -207 °C |
| Boiling point | -191.3 °C |
| Specific gravity (air = 1) | 0.968 |
| Density, gas* | 1.250 g/L |
| Density, liquid | 0.793 |
| Solubility At 0 °C At 25 °C |
3.54 mL/100 mL water 2.14 mL/100 mL water |
* Value indicated is at 0 °C, 101.3 kPa (760 mmHg). | |
1.6. Carbon Monoxide (CAS No. 630-08-0) Prevalence and Use With the single exception of CO2, the total yearly emissions of CO exceed all other atmospheric pollutants combined (5.13.). Some of the potential sources for CO emission and exposure are listed (5.13., 5.14.):
Foundries
Petroleum refineries
Fluid catalytic crackers
Fluid coking
operations
Moving-bed catalytic crackers
Kraft pulp mills
Carbon black manufacturers
Steel mills
Coke ovens
Basic oxygen furnaces
Sintering
operations
Formaldehyde manufacturers
Coal combustion facilities
Utility and large industrial boilers
Commercial and domestic
furnaces
Fuel oil combustion operations
Power plants
Industrial, commercial, and domestic
uses
Charcoal manufacturers
Meat smokehouses
Sugarcane
processing operations
Motor vehicles
1.7. Toxicology
(Information contained within this section is a synopsis of present knowledge of the physiological effects of CO and is not necessarily intended to be used as the basis for OSHA policy.)
Carbon monoxide has over a 200-fold greater affinity for hemoglobin
than has oxygen (5.15., 5.16.). Thus, it can make hemoglobin incapable
of carrying oxygen to the tissues. Also, the presence of
The signs and symptoms of CO poisoning include headache, nausea,
weakness, dizziness, mental confusion, hallucinations, cyanosis, and
depression of the
The uptake rate of CO by blood when air containing CO is breathed increases from 3 to 6 times between rest and heavy work. The uptake rate is also influenced by oxygen partial pressure and altitude (5.17.).
Carbon monoxide can be removed through the lungs when
Acute poisoning from brief exposure to high concentrations rarely
leads to permanent disability if recovery occurs. Chronic effects from
repeated exposure to lower concentrations have been reported. These
include visual and auditory disturbances and heart irregularities.
Where poisoning has been long and severe,
The following table gives the levels of
| Atmospheric CO (ppm) |
COHb in Blood (%) |
Symptoms |
|
| ||
| 70 | 10 | Shortness of breath upon vigorous exertion; possible tightness across the forehead. |
| 120 | 20 | Shortness of breath with moderate exertion; occasional headache with throbbing in the temples. |
| 220 | 30 | Decided headache; irritability; easily fatigued; disturbed judgment; possible dizziness; dimness of vision. |
| 350-520 | 40-50 | Headache; confusion; collapse; fainting upon exertion. |
| 800-1220 | 60-70 | Unconsciousness; intermittent convulsions; respiratory failure; death if exposure is prolonged. |
| 1950 | 80 | Rapidly fatal. |
|
| ||
Adults (non-smokers) normally have about 1%
In examining the CO levels in an occupational environment, consideration may also need to be made for CO generated from tobacco smoking. These amounts may ordinarily be small, but when added to the amounts generated by occupational activities, may aggravate conditions from an already existing high concentration of CO (5.19., 5.20.).
1.8. Other Hazardous Properties
Carbon monoxide is flammable and is a dangerous fire and explosion risk. The flammable limits in air range from 12 to 75% by volume (5.16.).
2. Sampling
2.1. Safety Precautions
2.1.1. Attach the sampling equipment to the worker in such a manner that it will not interfere with work performance or safety.
2.1.2. Follow all safety practices that apply to the work area being sampled.
2.2. Equipment
Note: The gas sample taken will contact the pump and tubing
during collection. The filter (if available) of the pump should be
clean and chemically inert to CO as well as any material inside the
pump that the sample comes in contact with. Pumps used to evaluate the
method were: Du Pont Model No.
2.2.1. Use a personal sampling pump capable of delivering a flow rate of approximately 0.01 to 0.05 L/min for TWA PEL samples. Use a larger flow rate pump (1 L/min) for Ceiling PEL measurements. Either pump must have an external inlet, an outlet port, and hose barbs.
2.2.2. Use five-layer aluminized gas sampling bags (5-L) as the
collection media (the bags can be obtained from the
2.2.3. Make pump, sampling media, and breathing zone connections with various lengths of flexible Tygon tubing.
2.3. Sampling Procedure
2.3.1. Calibrate the personal sampling pumps. Since the sampling bags have a total volume capacity of approximately 6 L, a sampling scheme for TWA PEL measurements is shown:
| Flow Rate (L/min) | Sampling Time (h) | Sample Vol(L) |
| 0.015 | 4 | 3.6 |
| 0.022 | 4 | 5.3 |
| 0.035 | 2.5 | 5.3 |
| 0.050 | 1.5 | 4.5 |
Take as large a sample as possible (<6 L) during the time
frame used for sampling. A large flow rate
2.3.2. Evacuate and check the gas sampling bags for leaks. Each sampling bag can be evacuated and leak tested by applying a vacuum to the bag. If a vacuum is applied to a leaky sampling bag, the bag will not fully collapse. If a vacuum pump is not available, inflate the gas sampling bags with nitrogen (N2), let them sit overnight, inspect for leaks, and then evacuate by hand rolling and flattening.
2.3.3. Label each sampling bag. Attach one end of a piece of
flexible tubing to the inlet hose barb of the pump, and place the
other end in the breathing zone of the worker. Use another piece of
tubing to connect the metal valve sampling bib of the sampling bag
to the outlet hose barb of the pump. A graphic representation of the
pump

2.3.4. For personal sampling, attach the gas sampling bag to any loose fitting clothing on the worker's back or side with tubing clamps.
2.3.5.When ready to sample, open the gas sampling bag valve by
rotating the metal valve
Note: If the employee being monitored is smoking a tobacco product during sampling, a positive contribution of CO from the combustion of tobacco may occur for personal samples. Ask the employee to refrain from smoking during sampling so that only the occupational exposure is measured.
2.3.6. After sampling, rotate the valve clockwise until tight.
Place an
2.3.7. Prepare samples and paperwork for submission to the laboratory. Do not prepare any blank samples. Request analysis for carbon monoxide.
2.3.8. When submitting sampling bags for analysis, pack loosely and pad generously to minimize potential damage during shipment. Submit samples to the laboratory as soon as possible after sampling.
3. Analysis
- Connect the outlet port of the personal sampling pump to the sampling loop via inert tubing.
- Adjust the pump to give a suitable flow rate for sample loading from the bag to the sampling loop.
- Connect a short piece of tubing from the inlet port of the pump to the sample bag. Turn the bag valve counterclockwise to the open position and turn on the pump.
- After the sample is loaded into the loop (which is vented to the atmosphere), turn off the pump to allow the loop sample to return to atmospheric pressure. Wait 1 to 2 min for pressure equalization and then open the gas sampling valve. Carrier gas flow is now directed through the sampling loop to the column and detector.
- Perform two determinations of each sample and standard.
3.1. Safety Precautions
3.1.1. Refer to instrument manuals and operating procedures for proper operation of the instruments.
3.1.2. Observe laboratory safety regulations and practices.
3.1.3. Prepare all CO standards in a well ventilated exhaust hood. AVOID inhaling CO.
3.2. Equipment
3.2.1. Instruments:
A GC fitted with a 1-mL stainless steel gas sampling loop, sampling valve, and DID is used. Loops other than 1 mL can also be used.
3.2.2. Standard media:
Five-layer aluminized gas sampling bags are used.
3.2.3. Columns:
A 4-foot × 1/8-inch stainless steel, 60-80 mesh, Hayesep Q column
and a
3.2.4. Data reduction:
An electronic integrator is used to calculate peak areas.
3.2.5. Standard generation:
Certified CO standards can be used or standards can be prepared
using any combination of: Calibrated
3.2.6. Additional accessories:
A personal sampling pump, with inlet and outlet ports and hose barbs, is used to load the gas sampling loop (loop loading can also be manually performed by squeezing the sampling bag).
3.3. Reagents (Gases)
3.3.1. A commercially prepared, bottled mixture of CO diluted
with either air or N2 is suitable for
generating gas standards. The CO concentration must be certified. If
a soap bubble flowmeter (~1 L/min) is used for standard preparation,
a mixture containing 100 ppm CO is convenient. If a
3.3.2. Filtered, compressed, CO-free air is used for dilutions when necessary. A convenient source of pure air is a cylinder of USP (United State Pharmacopeia) grade air. Small amounts of CO can be removed from the air by using a catalytic filter unit containing hopcalite to convert any CO to CO2.
3.3.3. Helium (research grade, <1 ppm impurities) is used as the carrier gas.
3.4. Standard Preparation
Prepare standards by either using a calibrated syringe or metered delivery of CO using flow measurement. When a soap bubble flow meter is used for gas flow measurements, apply water vapor corrections if necessary, since the gas flowing through the meter expands somewhat upon saturation with water vapor. As an example, consider the case where dry gas at 101.3 kPa pressure (760 mmHg) enters a flow meter and is saturated with water vapor [vapor pressure = 2.9 kPa (22 mmHg)]. In this case the gas volume (and therefore the gas flow rate) will be measured at (104.2/101.3 = 1.029) times the actual values. Specific cases of whether or not to use vapor corrections are given below.
Note: Commercially prepared standards in gas cylinders, if
available, can be used in place of
A standard generation scheme using 100-ppm CO with metered delivery is proposed as follows:
| Standard (ppm) | 100-ppm CO Volume (L) | Volume of Air (L) |
| Blank | 0.00 | 4.00 |
| 10 | 0.40 | 3.60 |
| 17 | 0.68 | 3.32 |
| 35 | 1.40 | 2.60 |
| 70 | 2.80 | 1.20 |
| 100 | 4.00 | 0.00 |
Other dilution schemes with different size gas bags and gas volumes can be used. For other concentrations of CO, use the following equation:
| ppm CO = A × | B
B + C |
Where:
A = CO concentration (ppm) in the
B = Pre-diluted CO
mixture volume (L),
C = Diluent air volume (L).
Note: % CO = ppm/10,000; i.e., if starting with a 0.50% CO mixture, A = 5,000 ppm.
Pure CO (A = 1 × 106) can also be used
for standard preparation. Prepare standards in concentrations that
bracket the sample concentrations. Always prepare a blank standard to
assure that the diluent air is not contaminated with CO. Completely
evacuate and flush the gas bags to be used for standard preparation
with
Note: All mixtures should be prepared within the confines of an exhaust hood.
3.4.1. Metered generations: Use a mass flow controller or
calibrated rotameter to verify and control the CO mixture delivery
rate from a gas cylinder. Use a soap bubble flowmeter before and
after the standard generation to verify the CO mixture flow rate.
Meter a known volume of
3.4.2. Syringe injections: Use a calibrated
3.5. Sample Preparation
No special preparations are necessary; however, the analyst should visually inspect the volume of the bags upon receipt and compare with the field air volumes in order to assess the possibility of leaks.
3.6. Sample Analysis
3.6.1. Recommended GC conditions:
Settings for a Tracor Model No. 540 GC and Model No. 706 DID are given in Appendix 1. Other settings may apply to different GCs.
3.6.2. Sample and standard introduction:
Note: Samples and standards can be introduced into the loop without a pump by simply squeezing a sufficient amount of sample from the bag into the loop. The sampling bag must be released for loop sample pressure normalization before opening the gas sampling valve.
3.6.3. Depending on column and GC flow characteristics, CO retention times are in the range of 12 to 14 min.
3.7. Interferences
The GC determination of CO is relatively specific; however, any compound having a similar column retention time as CO is a potential interference. Interferences can be minimized by altering operational conditions such as oven temperature and column packings. Using the conditions stated within this method, other common gases and vapors do not present serious potential interferences. Carbon dioxide is adsorbed on the molecular sieve column and does not interfere. Hydrogen, oxygen, nitrogen, methane, and carbon monoxide will elute in the order listed (5.10.). However, the CO peak will normally appear on the shoulder of the N2 peak for air; therefore, GC conditions should be set so that a distinct CO peak is obtained. A chromatogram showing the elution of CO in N2 is shown in Figure 1.
If necessary, the sample can be analyzed by
3.8. Calculations
3.8.1 If blank correction is necessary for the standards,
subtract the blank peak area from the standard area readings before
constructing the
3.8.2. Calculate CO concentrations from a
3.8.3. Report results to the industrial hygienist as ppm CO.
4. Backup Report
Experimental Protocol
The validation of the method consists of the following experimental protocol:
- Analysis of three sets of six spiked carbon monoxide (CO) samples having concentration ranges of approximately 0.5, 1, and 2 × TWA PEL.
- Analysis of three sets of six dynamically generated CO samples having concentration ranges of approximately 0.5, 1, and 2 × TWA PEL. Also, analysis of six generated samples having a concentration close to the Ceiling PEL value.
- Determination of the storage stability of CO samples collected in gas sampling bags.
- Determination of any variation in results when sampling at low and high humidity levels.
- Determination of the qualitative and quantitative detection limits for the analysis of CO.
- Comparison with a previous GC method used for CO determinations in which the CO was reduced to methane and analyzed with a flame ionization detector (FID).
- Assessment of the performance of this method and conclusions.
All samples, blanks, and standards used for validation were analyzed by
direct injection into a
OEi = ± [|mean biasi| + 2CVi] × 100%
where i is the respective sample pool being examined.
- Gas sampling bags were flushed several times with N2. A vacuum was then applied to completely collapse the bags.
- Air (USP grade) was used as a diluent after flowing through an
Ecolyzer No. 7915 Zero Air Filter. A known amount of air was
metered into each sampling bag. Compressed air flow rates were
measured before and after each bag filling using a soap bubble
flowmeter (Model
M-5, A. P. Buck, Inc., Orlando, FL). Air flow was regulated with aregulator-rotameter system. Blank samples of the compressed air were periodically collected and analyzed along with the samples and standards. - A known amount of CO was injected into each sampling bag containing diluent air using a calibrated gas syringe. A gas cylinder containing 0.50% CO in N2 (certified, Linde Div., Union Carbide Corp., Denver, Colorado) was used as the CO source.
- Standards were prepared by dilution of
104-ppm CO in N2 (certified, Airco, Inc., Murray Hill, NJ) with USP grade air.The CO content of the cylinder was confirmed employing a simplified modification of a method used by Grant, Katz, and Haines (5.24.). A known volume of the
104-ppm CO was passed through iodine pentoxide contained in a glass tube at about 150 °C. The resulting iodine was collected in an aqueous solution of potassium iodide. The amount of iodine formed was determined by titration using standard thiosulfate solution with starch as the indicator (5.25.). Two samples were collected. Carbon monoxide concentration determinations of 99.4 and 95.4 ppm (95.6% and 91.7% recovery respectively) were obtained. Previous work had demonstrated that the results for this procedure tend to be slightly lower than expected (5.24.). For the present work, the given concentration of 104 ppm was used. - Known amounts of CO and air were metered into gas sampling bags as described in Section 4.1.1. above.
- Gas sampling bags were flushed several times with N2. A vacuum was then applied to completely collapse the bags.
- A dynamic gas generation system was assembled as shown in
Figure 2. Moisture and other contaminants were removed from the
diluent air by using a charcoal/Drierite/silica gel filtering
system. A humidity, temperature, and flow control system (Model
HCS-301, Miller-Nelson Research Inc., Monterey, CA) was used to treat the diluent air to produce the stated RH at 25 °C. Diluent air flow was measured before and after each experiment using a dry test meter (ModelDTM-115, American Meter Co., Philadelphia PA). The flow control system was calibratedin-house for temperature and humidity prior to use. - The CO (0.5% in N2) was introduced
into the flow system via a glass mixing chamber. Gas flow rates
were taken immediately before and after each experiment using a
soap bubble flowmeter (Model
823-1, Mast Development Co., Davenport, IA). Flow rates were controlled using a mass flow controller (ModelFC-261, Tylan Corp., Torrance, CA).To assure continuous generation of controlled concentrations of CO and provide an additional verification of concentrations, the flow system was continuously monitored during each test with a
direct-reading instrument [Model 7140 (for CO), Interscan Corp., Chatsworth, CA] connected to the flow system. Calibration of the instrument was performed with a40-ppm calibrating gas (certified, Alphagaz, Cambridge, MD).
4.1. Analysis (spiked samples)
Procedure: Three sets of spiked samples were prepared and analyzed as follows:
4.1.1. Samples were prepared according to the following procedure:
4.1.2. Analytical standards were prepared according to the following procedure:
4.1.3. These samples and standards were analyzed within 4 days of preparation.
Results: Spiked sample recoveries (found/taken) are listed in Table 1. All analysis data passed the ASTM outlier test. The Bartlett's test valve (13.73) was high, most likely because the mean and standard deviation values at 1 × TWA PEL were exceptionally better than the other levels tested.
Note: When a set of results fails the Bartlett's test, possible options are to reject the results as being derived from significantly different sample populations, or adopt the CV at the PEL as the "Pooled CV". In this case the CV1 (Pooled) result was used instead. This appears more conservative since CV1 (Pooled) > CV1 (TWA PEL).
The data (Table 1) indicated good precision and accuracy. The CV1 was 0.038 and the average analytical recovery was 98.1%.
4.2. Sampling and Analysis (Generated samples)
Procedure: Three sets of generated samples at 0.5, 1, and 2 × TWA PEL were prepared and analyzed.
4.2.1. Samples were prepared according to the following procedure:
4.2.2. Samples were analyzed within 2 days after preparation; standards were used within 4 days after preparation.
4.2.3. Six samples with a CO concentration near the Ceiling PEL (200 ppm) were also generated using the system described above. Standards were prepared by injection of CO (0.5% in N2) using a calibrated gas syringe into a gas bag containing a known volume of USP grade air. Samples and standards were analyzed 4 days after preparation.
Results: Recoveries for generated samples at 0.5, 1, and 2 × TWA PEL are listed in Table 2a. The Sampling and Analysis date showed good precision and accuracy. All data passed the outlier test. The Bartlett's test value (9.92) was slightly higher than the critical value (9.23), again probably due to greater precision for the TWA PEL results. The data were pooled, since it was felt a significant amount of error would not be introduced by pooling. The CV2 (Pooled) value is similar to that found during the humidity studies (Section 4.4.).
Recoveries for the samples generated at the Ceiling PEL level are given in Table 2b. These results showed excellent recovery.
The results are summarized as follows:
| PEL | Ave. Recov. (%) | CV |
| TWA | 105.8 | 0.020 |
| Ceiling | 100.0 | 0.025 |
4.3. Stability Test
Procedure: A long-term evaluation of sample media
stability was performed to determine any potential problems if delays
in sample analyses occur.
Samples were also generated at 0.5 × TWA PEL and 80% RH; however, a few of the bags used for this experiment appeared to have some leakage and technical problems occurred during analysis.
Results: Recovery data are listed in Table 3 and graphically represented in Figure 3 (normalized data). One result for 1 × PEL at 8 days of storage is not included in Figure 3 since it appeared to be unrealistically high; The GC appeared to display some instability on the day these samples were analyzed.
Previously, different types of gas sampling bags were evaluated for
stability, structural integrity, and compactness. The
A summary of the stability data from the GC analysis is shown below:
| 1 × TWA PEL |
2 × TWA PEL | |||||
| Day | Recovery* | CV | Day | Recovery* | CV | |
| 1 | 100.0 | 0.023 | 2 | 100.0 | 0.041 | |
| 8 | 103.5 | 0.161 | 15 | 89.6 | 0.108 | |
| 21 | 80.9 | 0.135 | 22 | 75.9 | 0.136 | |
| 29 | 81.9 | 0.141 | 32 | 79.0 | 0.211 | |
| 39 | 79.9 | 0.176 | ||||
| * Normalized to 100% | ||||||
The slope of the plotted normalized 1 × PEL data is 0.00597
4.4. Humidity Study
Procedure: Samples were also generated at
Results: The results of the gas sampling bags
collected at the three RH are presented in Tables 2 (50% RH) and 4
4.5. Detection Limits
Procedure: Both qualitative and quantitative limits for the analysis of CO by GC were calculated using the International Union of Pure and Applied Chemistry (IUPAC) method for detection limit determinations (5.26.). The procedure used for determining the detection limits is as follows:
4.5.1. Gas bags were prepared as described in Section 4.1.1., Step 1.
4.5.2. Blank samples were generated using the flow, humidity, and temperature control system mentioned in Section 2.
4.5.3. Low concentration CO samples were prepared by mixing CO (0.50% in N2), via the mixing chamber, with the treated air. Concentrations of 1.70, 2.62, 5.13, and 10.16 ppm were used.
Results: Detection limit results are listed in Table
5. The qualitative detection limit was 0.12 ppm. The quantitative
detection limit was 0.40 ppm. A
4.6. Comparison Methods
The results obtained in the present study were compared with those
obtained during the CO detector tube evaluation study (5.5.). For the
detector tube study, the CO atmospheres generated were sampled
4.7. Method Performance - Conclusions
The data generated during the validation of the method indicate an
acceptable method for sampling and analyzing CO. The
Obtaining good analytical results appears to be contingent on analyzing the samples within two weeks, and preferable as soon as possible after collection. Storage stability appears to be enhanced if a large gas sample (4 L or more) is taken. It is also possible that storage stability may be improved if newer bags are used which are free of small leaks and internal deposits. Gas bag samples should be sent to the laboratory and analyzed as soon as possible.
This method is capable of accurate and precise measurements to
determine compliance with the
5. References
5.1. "Air Contaminants; Final Rule" Federal Register 29 CFR
Part 1910 (19 Jan. 1989). Pp.
5.2. United States Department of Labor, OSHA: "Memorandum, Updated Changes to 29 CFR 1910.1000, Air Contaminants Standard." by Patricia Clark, Director Designate, Directorate of Compliance Programs. United States Department of Labor, OSHA, Washington, DC, June 1, 1990. [Memo].
5.3. Directorate of Technical Support, OSHA-DOL: Chemical
Information File, Online
5.4. Katz, M., ed.: Methods of Air Sampling and Analysis. 2nd ed., APHA Intersociety Committee. Washington, D.C.: Publication Office, American Public Health Association, 1977. No. 132, pp. 368-369.
5.5. Occupational Safety and Health Administration Salt Lake
Technical Center: Carbon Monoxide Detector Tubes
5.6. National Institute for Occupational Safety and Health:
NIOSH Manual of Analytical Methods. 2nd. ed., Vol. 1 (Method
No. P&CAM 112) (DHEW/NIOSH Pub. No.
5.7. National Institute for Occupational Safety and Health:
NIOSH Manual of Analytical Methods. 2nd. e., Vol. 4 (Method No. S340)
(DHEW/NIOSH Pub. No.
5.8. Mine Safety and Health Administration: Regular Mine Gas Analysis (MSHA Standard Method No. 1). Denver, CO: MSHA, 1979.
5.9. Guiochon, G. and C. Pommier: Gas Chromatography in
Inorganics and Organometallics. Ann Arbor, MI: Ann Arbor Science
Publishers Inc., 1973. pp.
5.10. Williams, D.M.: "Unique Applications for New Helium Glow Discharge Ionization Detector for Gas Chromatography." Paper presented at the 1988 Pittsburgh Conference, New Orleans, LA, February 1988.
5.11. American Society of Heating, Refrigerating and
5.12. Sax, N.I.: Dangerous Properties of Industrial
Materials. 4th ed. New York: Van Nostrand Reinhold Company, 1975. pp.
5.13. National Institute for Occupational Safety and Health:
Criteria for a Recommended Standard--Occupational Exposure to
Carbon Monoxide (DHEW/NIOSH Pub. HSM
5.14. Coburn, R.F., chairman: Carbon Monoxide. Washington, DC: National Academy of Sciences, 1977.
5.15. Proctor, N.H. and J.P. Hughes: Chemical Hazards of
the Workplace. Philadelphia, PA: J.B. Lippincott Co., 1978. pp.
5.16. Sax, N.I. and R.J. Lewis, Sr.: Hawley's Condensed
Chemical Dictionary. 11th ed. New York: Van Nostrand Reinhold Co.,
1987. pp.
5.17. American Conference of Governmental Industrial
Hygienists: Documentation of the Threshold Limit Values for
Substances in Workroom Air. 3rd ed. Cincinnati, OH: American
Conference of Governmental Industrial Hygienist, 1971. pp.
5.18. Ellenhorn, M.J., and D.G. Barceloux: Medical Toxicology. New York, NY: Elsevier Science Publishing Co., Inc., 1988. p. 821.
5.19. Lee, H.K., T.A. McKenna, L.N. Renton, and J. Kirbride:
Impact of a New Smoking Policy on Office Air Quality. In Indoor Air
Quality in Cold Climates: Hazards and Abatement Measures, edited
by D.S. Walkinshaw. Pittsburgh PA: Air Pollution Control Association.
pp.
5.20. Leaderer, B.P., W.S. Cain, R. Isseroff, and L.G.
Berglung: Ventilation Requirements in Buildings. II. Particulate
Matter and Carbon Monoxide from Cigarette Smoking. Atmospheric
Environment 18, No. 1:
5.21. Mandel, J.: Accuracy and Precision, Evaluation and
Interpretation of Analytical Results, The Treatment of Outliers. In
Treatise On Analytical Chemistry. 2nd ed., Vol. 1, edited by
I.M. Kolthoff and P.J. Elving. New York, NY: John Wiley and Sons,
1978. pp.
5.22. National Institute for Occupational Safety and Health:
Documentation of the NIOSH Validation Tests by D. Taylor, R.
Kupel and J. Bryant (DHEW/NIOSH Pub. No.
5.23. Occupational Safety and Health Administration Salt Lake
Technical Center: OSHA Analytical Methods Manual. Vol. III
5.24. Grant, G.A., M. Katz, and R.L. Haines: A Modified
Iodine Pentoxide Method for the Determination of Carbon Monoxide. Can.
J. of Technol. 29:
5.25. Skoog, D.A. and D.M. West: Analytical Chemistry: an
Introduction. 4th ed. Philadelphia, PA: Saunders College
Publishing, 1986. pp.
5.26. Long, G.L. and J.D. Winefordner: Limit of
| Analysis
Spiked CO Samples | |||||||
| (OSHA TWA PEL) | |||||||
| PPM Taken |
PPM Found |
F/T | N | Mean | Std Dev | CV | OE |
| (0.5 × PEL) | |||||||
| 17.600 | 17.741 | 1.008 | |||||
| 17.600 | 16.274 | 0.925 | |||||
| 17.600 | 15.691 | 0.892 | |||||
| 17.600 | 17.889 | 1.016 | |||||
| 17.500 | 17.326 | 0.990 | |||||
| 17.500 | 15.684 | 0.896 | |||||
| 6 | 0.954 | 0.057 | 0.060 | 16.5 | |||
| (1 × PEL) | |||||||
| 35.200 | 34.619 | 0.983 | |||||
| 35.200 | 34.353 | 0.976 | |||||
| 35.200 | 34.523 | 0.981 | |||||
| 35.300 | 34.346 | 0.973 | |||||
| 35.300 | 34.080 | 0.965 | |||||
| 35.500 | 34.254 | 0.965 | |||||
| 6 | 0.974 | 0.008 | 0.008 | 4.2 | |||
| (2 × PEL) | |||||||
| 70.200 | 71.064 | 1.012 | |||||
| 70.000 | 67.827 | 0.969 | |||||
| 69.800 | 72.870 | 1.044 | |||||
| 70.000 | 73.249 | 1.046 | |||||
| 70.100 | 70.539 | 1.006 | |||||
| 70.000 | 70.405 | 1.006 | |||||
| 6 | 1.014 | 0.029 | 0.028 | 7.0 | |||
| F/T = Found/Taken | OE | = Overall Error (±%) | |||||
| Bias | = -0.019 | ||||||
| CV1 (Pooled) | = 0.038 | ||||||
| Overall Error (Total) | = ±9.6% | ||||||
| Sampling and Analysis
50% RH and 25 °C | |||||||
| (OSHA TWA PEL) | |||||||
| PPM Taken |
PPM Found |
F/T | N | Mean | Std Dev | CV | OE |
| (0.5 × PEL) | |||||||
| 17.200 | 18.181 | 1.057 | |||||
| 17.200 | 19.160 | 1.114 | |||||
| 17.200 | 18.729 | 1.089 | |||||
| 17.200 | 19.082 | 1.109 | |||||
| 17.200 | 18.531 | 1.077 | |||||
| 17.200 | 17.570 | 1.021 | |||||
| 6 | 1.078 | 0.035 | 0.032 | 14.2 | |||
| (1 × PEL) | |||||||
| 30.800 | 34.305 | 1.114 | |||||
| 30.800 | 33.635 | 1.092 | |||||
| 30.800 | 33.746 | 1.096 | |||||
| 30.800 | 33.884 | 1.100 | |||||
| 30.800 | 34.176 | 1.110 | |||||
| 30.800 | 34.019 | 1.105 | |||||
| 6 | 1.103 | 0.008 | 0.008 | 11.8 | |||
| (2 × PEL) | |||||||
| 63.600 | 62.072 | 0.976 | |||||
| 63.600 | 63.479 | 0.998 | |||||
| 63.600 | 63.783 | 1.003 | |||||
| 63.600 | 63.860 | 1.004 | |||||
| 63.600 | 63.100 | 0.992 | |||||
| 63.600 | 62.426 | 0.982 | |||||
| 6 | 0.992 | 0.012 | 0.012 | 3.1 | |||
| F/T = Found/Taken | OE | = Overall Error (±%) | |||||
| Bias | = +0.058 | ||||||
| CV2(Pooled) | = 0.020 | ||||||
| CVT(Pooled) | = 0.025 | ||||||
| Overall Error (Total) | = ±10.8% | ||||||
| Ceiling PEL Study
50% RH and 25 °C | |||||||
| PPM Taken |
PPM Found |
F/T | N | Mean | Std Dev | CV | OE |
| 197.500 | 199.565 | 1.010 | |||||
| 197.500 | 201.207 | 1.019 | |||||
| 197.500 | 200.182 | 1.014 | |||||
| 197.500 | 201.349 | 1.019 | |||||
| 197.500 | 192.328 | 0.974 | |||||
| 197.500 | 190.057 | 0.962 | |||||
| 6 | 1.000 | 0.025 | 0.025 | 5.0 | |||
| F/T = Found/Taken | OE | = Overall Error (±%) | |||||
| Storage Stability Test | ||||||||
| 1 × TWA PEL, 80% RH | ||||||||
| Storage | PPM Taken |
PPM Found |
N | Mean | Std Dev | CV | Recov. % |
Normalized to 100% |
| Day 1 | 31.600 | 34.718 | ||||||
| 31.600 | 34.612 | |||||||
| 31.600 | 32.851 | |||||||
| 31.600 | 33.680 | |||||||
| 31.600 | 32.993 | |||||||
| 31.600 | 33.905 | |||||||
| 6 | 33.793 | 0.785 | 0.023 | 106.9 | 100.0 | |||
| Day 8* | 31.600 | 43.028 | ||||||
| 31.600 | 30.716 | |||||||
| 31.600 | 36.747 | |||||||
| 31.600 | 26.702 | |||||||
| 31.600 | 36.089 | |||||||
| 31.600 | 36.484 | |||||||
| 6 | 34.961 | 5.623 | 0.161 | 110.6 | 103.5 | |||
| Day 21 | 31.600 | 25.315 | ||||||
| 31.600 | 25.128 | |||||||
| 31.600 | 29.057 | |||||||
| 31.600 | 22.758 | |||||||
| 31.600 | 28.697 | |||||||
| 31.600 | 33.142 | |||||||
| 6 | 27.350 | 3.700 | 0.135 | 86.5 | 80.9 | |||
| Day 29 | 31.600 | 28.268 | ||||||
| 31.600 | 27.629 | |||||||
| 31.600 | 29.594 | |||||||
| 31.600 | 20.039 | |||||||
| 31.600 | 29.396 | |||||||
| 31.600 | 31.012 | |||||||
| 6 | 27.656 | 3.910 | 0.141 | 87.5 | 81.9 | |||
| Day 39 | 31.600 | 25.872 | ||||||
| 31.600 | 25.789 | |||||||
| 31.600 | 29.632 | |||||||
| 31.600 | 18.690 | |||||||
| 31.600 | 30.118 | |||||||
| 31.600 | 31.884 | |||||||
| 6 | 26.998 | 4.739 | 0.176 | 85.4 | 79.9 | |||
| * | Plot of standards showed more than the
usual scatter-- GC performance was erratic. | |||||||
| Storage Stability Test | ||||||||
| 2 × TWA PEL, 80% RH | ||||||||
| Storage | PPM Taken |
PPM Found |
N | Mean | Std Dev | CV | Recov. % |
Normalized to 100% |
| Day 2 | 76.200 | 68.643 | ||||||
| 76.200 | 68.459 | |||||||
| 76.200 | 69.570 | |||||||
| 76.200 | 74.060 | |||||||
| 76.200 | 74.882 | |||||||
| 76.200 | 69.094 | |||||||
| 6 | 70.785 | 2.893 | 0.041 | 92.9 | 100.0 | |||
| Day 15 | 76.200 | 60.295 | ||||||
| 76.200 | 67.145 | |||||||
| 76.200 | 70.047 | |||||||
| 76.200 | 69.268 | |||||||
| 76.200 | 61.652 | |||||||
| 76.200 | 51.995 | |||||||
| 6 | 63.400 | 6.862 | 0.108 | 83.2 | 89.6 | |||
| Day 22 | 76.200 | 47.042* | ||||||
| 76.200 | 55.069 | |||||||
| 76.200 | 65.320* | |||||||
| 76.200 | 55.451 | |||||||
| 76.200 | 54.587 | |||||||
| 76.200 | 44.728 | |||||||
| 6 | 53.700 | 7.288 | 0.136 | 70.5 | 75.9 | |||
| Day 32 | 76.200 | 45.644* | ||||||
| 76.200 | 59.314 | |||||||
| 76.200 | 69.860* | |||||||
| 76.200 | 67.860 | |||||||
| 76.200 | 52.431 | |||||||
| 76.200 | 40.675 | |||||||
| 6 | 55.964 | 11.820 | 0.211 | 73.4 | 79.0 | |||
| * | Bag contents were somewhat low, indicating leakage. | |||||||
| Humidity Study
25-28% RH and 25 °C | |||||||||
| (OSHA TWA PEL) | |||||||||
| PPM Taken |
PPM Found |
F/T | N | Mean | Std Dev | CV | OE | ||
| (0.5 × PEL) | |||||||||
| 17.700 | 21.592 | 1.220 | |||||||
| 17.700 | 20.188 | 1.141 | |||||||
| 17.700 | 19.368 | 1.094 | |||||||
| 17.700 | 20.083 | 1.135 | |||||||
| 17.700 | 20.681 | 1.168 | |||||||
| 17.700 | 20.840 | 1.177 | |||||||
| 6 | 1.156 | 0.043 | 0.037 | 23.0 | |||||
| (1 × PEL) | |||||||||
| 31.100 | 30.248 | 0.973 | |||||||
| 31.100 | 31.445 | 1.011 | |||||||
| 31.100 | 31.719 | 1.020 | |||||||
| 31.100 | 31.081 | 0.999 | |||||||
| 31.100 | 32.577 | 1.048 | |||||||
| 31.100 | 31.972 | 1.028 | |||||||
| 6 | 1.013 | 0.026 | 0.025 | 6.4 | |||||
| (2 × PEL) | |||||||||
| 62.900 | 60.486 | 0.962 | |||||||
| 62.900 | 59.319 | 0.943 | |||||||
| 62.900 | 55.289 | 0.879 | |||||||
| 62.900 | 59.345 | 0.943 | |||||||
| 62.900 | 61.934 | 0.985 | |||||||
| 62.900 | 59.764 | 0.950 | |||||||
| 6 | 0.944 | 0.035 | 0.037 | 13.1 | |||||
| F/T = Found/Taken | OE | = Overall Error (±%) | |||||||
| Bias | = +0.038 | ||||||||
| CV (Pooled) | = 0.034 | ||||||||
| Overall Error (Total) | = ±10.5% | ||||||||
| Humidity Study 80% RH and 25 °C | |||||||||
| (OSHA TWA PEL) | |||||||||
| PPM Taken |
PPM Found |
F/T | N | Mean | Std Dev | CV | OE | ||
| (0.5 × PEL) | |||||||||
| 17.900 | 18.342 | 1.025 | |||||||
| 17.900 | 18.943 | 1.058 | |||||||
| 17.900 | 18.177 | 1.015 | |||||||
| 17.900 | 18.027 | 1.007 | |||||||
| 17.900 | 18.590 | 1.039 | |||||||
| 17.900 | 17.358 | 0.970 | |||||||
| 6 | 1.019 | 0.030 | 0.030 | 7.8 | |||||
| (1 × PEL) | |||||||||
| 31.600 | 34.718 | 1.099 | |||||||
| 31.600 | 34.612 | 1.095 | |||||||
| 31.600 | 32.851 | 1.040 | |||||||
| 31.600 | 33.680 | 1.066 | |||||||
| 31.600 | 32.993 | 1.044 | |||||||
| 31.600 | 33.905 | 1.073 | |||||||
| 6 | 1.069 | 0.025 | 0.023 | 11.6 | |||||
| (2 × PEL) | |||||||||
| 76.200 | 68.643 | 0.901 | |||||||
| 76.200 | 68.459 | 0.898 | |||||||
| 76.200 | 69.570 | 0.913 | |||||||
| 76.200 | 74.060 | 0.972 | |||||||
| 76.200 | 74.882 | 0.983 | |||||||
| 76.200 | 69.094 | 0.907 | |||||||
| 6 | 0.929 | 0.038 | 0.041 | 15.3 | |||||
| F/T = Found/Taken | OE | = Overall Error (±%) | |||||||
| Bias | = +0.006 | ||||||||
| CV (Pooled) | = 0.032 | ||||||||
| Overall Error (Total) | = ±7.0% | ||||||||
| F Test |
Recoveries % | ||||||
| Level | F(calc) | F(crit) | RH | 25-28% | 50% | 80% | |
| 0.5 × PEL | 21.44 | 6.36 | 115.6 | 107.8 | 101.9 | ||
| 1.0 × PEL | 27.52* | 6.36 | 101.3 | 110.3 | 106.9 | ||
| 2.0 × PEL | 7.02* | 6.36 | 94.4 | 99.2 | 92.9 | ||
| Average | 103.8 | 105.8 | 100.6 | ||||
| Large values appear to be due to variability in sample generation and not to any significant humidity effect. |
| Determination of Qualitative and Quantitative Detection Limits | |||||||||
| PPM |
Integrated Area |
||||||||
| Blank* | 1,746 | 1,601 | 1,649 | 1,769 | 1,634 | 1,630 | 68.8 | ||
| 1.70* | 5,281 | 5,254 | 5,604 | 5,659 | 5,686 | 211.6 | |||
| 2.62 | 7,992 | 6,602 | 8,214 | 8,032 | 8,323 | 7,342 | 658.2 | ||
| 5.13 | 10,213 | 11,142 | 10,511 | 9,054 | 10,091 | 10,066 | 681.9 | ||
| 10.16 | 19,305 | 22,274 | 18,974 | 19,903 | 18,917 | 20,201 | 1,256.9 | ||
| * | Manual integration was performed on
chromatographic peaks using CPLOT software
| ||||||||
| IUPAC Method | |||
| Using the equation: | Cld = k(sd)/m | ||
| Where: | |||
| Cld | = | the smallest detectable concentration an analytical instrument can determine at a given confidence level. | |
| k | = | 3 (Qualitative detection limit, 99.86% confidence). | |
| = | 10 (Quantitative detection limit, 99.99% confidence). | ||
| sd | = | standard deviation of blank readings. | |
| m | = | analytical sensitivity or slope as calculated by linear regression. | |
| Minimum detectable signal (Qualitative detection limit): | |||
| Cld = 3(68.8)/1,718.5 | |||
| Cld = 0.12 ppm | |||
| For k = 10(Quantitative detection limit): | |||
| Cld = 0.40 ppm as a reliable detectable signal | |||
| Method Comparison
Analysis of Gas Bags Containing CO by GC Using a | |||||
| Test | Samples | Mean Recov.** | CV | OET%*** | |
| %RH | × PEL | N* | |||
| 25-30 | 0.5 | 3 | 0.944 | 0.004 | 8.2 |
| 1 | 4 | 0.937 | 0.018 | ||
| 2 | 3 | 0.993 | 0.028 | ||
| 50 | 0.5 | 3 | 0.933 | 0.006 | 6.0 |
| 1 | 3 | 1.028 | 0.011 | ||
| 2 | 6 | 0.987 | 0.028 | ||
| 80 | 0.5 | 4 | 1.013 | 0.007 | 5.3 |
| 1 | 6 | 1.000 | 0.037 | ||
| 2 | 6 | 0.988 | 0.017 | ||
| Test | Samples | Mean Recov.** | CV | OET%*** | |
| %RH | × PEL | N* | |||
| 25-30 | 0.5 | 6 | 1.156 | 0.037 | 10.5 |
| 1 | 6 | 1.013 | 0.025 | ||
| 2 | 6 | 0.944 | 0.037 | ||
| 50 | 0.5 | 6 | 1.078 | 0.032 | 9.8 |
| 1 | 6 | 1.103 | 0.008 | ||
| 2 | 6 | 0.992 | 0.012 | ||
| 80 | 0.5 | 6 | 1.019 | 0.030 | 7.0 |
| 1 | 6 | 1.069 | 0.023 | ||
| 2 | 6 | 0.929 | 0.041 | ||
| * | These samples were collected throughout the detector tube sampling period (5.5.). | ||||
| ** | Results were compared to standards prepared from
| ||||
| *** | Note: OET% (Total Overall Error in %) is the pooled result of all three concentrations at one RH level. | ||||
| Precision and Accuracy Summary | ||||
| Precision: | CV1(Pooled) = 0.038 | |||
| CV2(Pooled) = 0.020 | ||||
| CVT(Pooled) = 0.025 | ||||
| Bartlett's Test | ||||
| Level | B(calc) | B(crit) | ||
| Spiked | ||||
| (Table 1) | 13.73 | 9.23 | ||
| Generated | ||||
| (Tables 2 and 4) | ||||
| 25-30% RH | 0.82 | 9.23 | ||
| 50% RH | 9.92 | 9.23 | ||
| 80% RH | 1.51 | 9.23 | ||
| Recovery: | Average recovery (Sampling and Analysis) = 105.8% | |||
| Average recovery (Ceiling PEL study) = 100.0% | ||||
| Mean Recovery, present GC method | 92.9 - 115.6% |
| CV, present GC method | 0.008 - 0.041 |
| Bias | 0.006 - 0.058 |
| CV2 (Pooled) | 0.020 - 0.034 |
| Mean Recovery, previous GC method** | 93.0 - 102.9% |
| CV, previous GC method** | 0.004 - 0.037 |
| * Range--includes different RH and concentration levels. | |
| ** GC method--methanizer and FID analysis. | |
| Analysis Parameters for CO Determinations | |||
| Gas chromatograph | Tracor Model No. 540 GC | ||
| Detector | Discharge ionization detector* | ||
| DID power supply | Tracor Model No. 706 | ||
| Polarizing voltage setting | 700 | ||
| Discharge current setting | 700 | ||
| Electrometer settings | |||
| Input | 10 | ||
| Output | 2 | ||
| GC temperature settings (°C) | |||
| Column oven | 90 | ||
| Valve oven | 60 | ||
| Detector | 190* | ||
| Flow control | 40 | ||
| Column pressure (kPa) | 50-70 | ||
| " " (psi) | 7-10 | ||
| Time settings (min) | |||
| Run time | 20.0 | ||
| Pre ready | 1.0 | ||
| On event | 0.01 | ||
| Off event | 0.30 | ||
| Columns (in series) | |||
| First | 4' × 1/8" SS Hayesep Q 60-80 (Mounted in valve oven) | ||
| Second | 12' × 1/8" SS Molecular Sieve 60-80 (Mounted in column oven) | ||
| Helium flow rates (L/min) | |||
| Through discharge | 0.030 | ||
| Through columns | 0.020 | ||
| Gas sampling loop volume (mL) | 1 | ||
| Integrator | Hewlett-Packard 3357 Laboratory Automation System (Rev. 2540)** | ||
| Recorder | Omniscribe Model No. B5218-5 | ||
| Y1 full scale setting (v) | 0.01 (TWA PEL), 0.1 (Ceiling PEL) | ||
| Chart speed (in/min) | 0.05 | ||
| CO peak time (min) | 12.0-14.6 | ||
| * | When the detector reaches operating temperature after startup, helium should be allowed to flow through the detector for one day before the discharge is started (manufacturer's recommendation). | ||
| ** | Area counts for CO concentrations of <2.5 ppm were not automatically integrated. These areas were determined by manual integration. | ||
Notes:
If instabilities develop in the chromatogram when the first portion of the gas sample (i.e., O2 and N2 peaks) pass through the DID, the GC may be programmed so that the gas flow from the columns is temporarily vented to the outside of the GC and does not pass through the DID. This venting would occur for about the first 8 or 10 min of the analysis, since the CO peak would normally occur at 12 to 15 min.
An early model of the DID which was used by our laboratory for this validation was prone to exhibit oscillatory behavior, requiring extensive down time and manufacturer service.
Chromatogram of Elution of 104 ppm CO in
N2 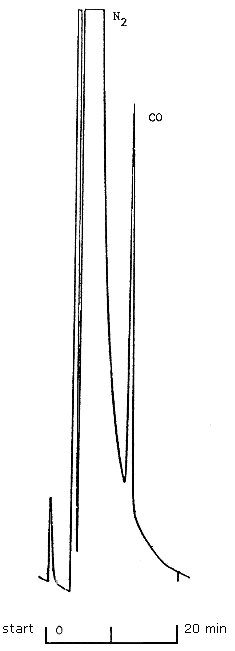
Figure 1
Dynamic Generation System for
Production of Carbon
Monoxide Atmospheres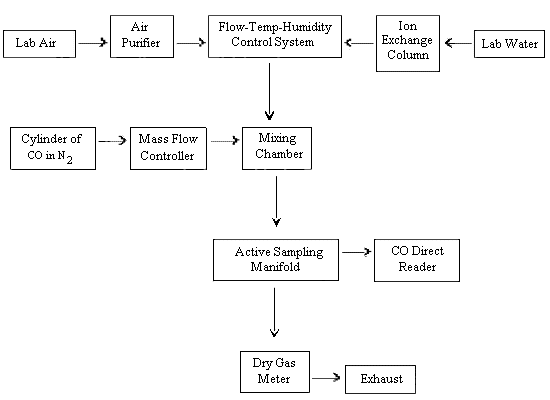
Figure 2
Storage Stability Study of CO in Gas Bags (1,2 × TWA
PEL)
Figure 3