NITROGEN DIOXIDE BACKUP DATA REPORT (ID-182)
This backup report was revised May, 1991
Introduction
The general procedure for the air sample collection and analysis of
nitrogen dioxide (NO2) is described in OSHA
Method No.
This method was evaluated when the Permissible Exposure Limit (PEL) was
a 5 ppm Ceiling. The OSHA Final Rule PEL for NO2
is currently 1 ppm. A
Generation System
All generation of nitrogen dioxide test atmospheres, and hence all experiments, with one exception, were performed using the equipment shown in Figure 1. The detection limit study did not use a test atmosphere generation for sample spiking and collection. Instead, samples were spiked with solutions of sodium nitrite.
Nitrogen dioxide permeation tubes (Thermedics Inc., Woburn, MA) were used as the contaminant source for all of the generation experiments except the conversion factor experiment. A cylinder of nitric oxide (NO) in nitrogen and oxidizer tubes were used to determine conversion factors. Permeation rates during the other experiments were determined by measuring the weight loss of three permeation tubes over a given period of time. A constant temperature of 35 °C was used. As shown below, the calculated overall NO2 permeation rate for the three tubes was 89.09 µg/min.
| Time Elapsed (min) |
Weight Loss (µg) |
Diffusion Rate (µg/min) |
|
| ||
| 4,335 5,700 10,105 10,095 |
387,600 513,400 904,200 882,100 |
89.41 90.07 89.48 87.38 |
| Ave. 89.09 ± 1.17 µg/min | ||
The NO2 produced from the permeation source
was diluted with a small amount of filtered air and then mixed, using a
glass mixing chamber, with filtered, tempered air. A flow, temperature and
humidity control system
Sampling Media
Three different
- NO2-NO collection device (Cat. No.
226-40, SKC, Eighty Four, PA):
The sampling device consists of three separate glass tubes, twoTEA-IMS tubes and an oxidizer tube. Each glass tube is flame sealed. Both sample collection tubes consist of 400 mgTEA-IMS. The oxidizer contains approximately 1 g of a chromate compound. EitherTEA-IMS tube can be used separately to monitor NO2. When sampling for both NO and NO2, the three tubes are connected with Tygon tubing such that the oxidizer tube is placed between the twoTEA-IMS sampling tubes. The tubes used during the experiments were from lot no. 374. - SKC combination tube (Cat. No.
226-40 discontinued, SKC):
This combination tube contained all three sections in a single tube. Two 400 mg sections ofTEA-IMS were separated by a 800 mg oxidizer section. This tube has been discontinued by SKC and replaced with the device mentioned above. The tubes used were from lot no. 306. - Supelco combination tube (Supelco, Bellefonte, PA):
This tube is similar in construction to the SKC lot no. 306 sampling tube (2) listed above with one exception. The Supelco tube uses a smaller mesh size of molecular sieve. Lot no.582-99 was used for a Sampling and Analysis experiment.
SKC collection devices (1) and (2) listed above are identical except device (1) has a physical segregation of sorbents and oxidizer.
Due to low recoveries found during a preliminary study with Supelco sampling tubes, these tubes were excluded from the experiments.
Sample Collection
Air samples were collected from the Teflon manifold using calibrated Du
Pont model P125 low flow pumps (flow rates of
Sample Analysis
Samples prepared for
Evaluation
The following experiments were performed for the evaluation of Method
No.
- Analysis - (DE) of spiked samples
- Sampling and Analysis - generation and analysis of NO2 samples
- Collection efficiency and breakthrough of
TEA-IMS sampling tubes - Storage stability of sampling tubes
- Sampling at different humidities
- Analytical method comparison
- Analytical detection limit determinations
- Determination of conversion factor for NO2
concentrations of 10 to 200 ppm.
The preliminary sampling and analysis experiment using Supelco tubes is discussed in Section 9.
A statistical protocol (11.6.) was used to evaluate results. Data were subjected to the Bartlett's (11.7.) and an Outlier test (11.8.) to determine homogeneity of variance and identify any extraneous data.
1. Analysis (Desorption Efficiency, DE)
Procedure: A total of 20 spiked samples (8 samples at 0.5
and 6 samples at 1 and 2 times the Transitional PEL) were prepared and
analyzed. Samples were prepared by spiking known amounts of
NO2 gas into
- 1.1. SKC lot no. 374 sampling tubes were used.
1.2. Known NO2 gas concentrations were
prepared by using a
Results: The results of the analysis study are presented in Table 1. All data passed the Bartlett's test. One result tested as an outlier and was omitted. Results were pooled. The data (Table 1) indicates acceptable precision and accuracy (11.6.) for the analytical portion of the method and does not indicate a need for a desorption correction factor. The coefficient of variation for analysis (CV1) was 0.021 and the average analytical or spiked recovery was 106%.
2. Sampling and Analysis
Procedure: A total of 18 samples (6 samples at each of the three test levels) were collected from dynamically generated test atmospheres and analyzed. Generation and analysis of NO2 was the same as mentioned in the Introduction. Sample results from the dynamic generation provide the overall error and precision of the sampling and analytical method. Overall error should be ±25% and was calculated using the following equation (11.6.):
Overall error = ± [ | mean bias | + 2CVT ] × 100%
- 2.1. SKC sampling tubes, lot no. 306, were used for this
experiment.
2.2. Samples were taken for
Results: The results of the sampling and analysis experiment are shown in Table 2. The sampling and analysis data also show acceptable precision and accuracy (11.6.). All data passed both the outlier and Bartlett's test and results were pooled. The pooled coefficients of variation for spiked CV1 (pooled), generated CV2 (pooled) samples, as well as the overall CVT (pooled), are as follows:
CV1 (pooled) = 0.021 CV2 (pooled) = 0.033 CVT (pooled) = 0.034
The overall bias was 13% high. Overall error was acceptable (< ±25%) and was ±19.8%.
3. Collection Efficiency and Breakthrough
- 3.1. Collection Efficiency
Procedure: Samples were generated to measure the sorbent collection efficiency at about 9.5 ppm NO2.
- 3.1.1. SKC sampling tubes, lot no. 306, were used to collect the
NO2 at 50% RH and 25 °C. These were the
combination tubes; each glass tube contained two sections of
3.1.2. Using the same generation system described in the Introduction, six samples were collected at 2 times the OSHA Transitional PEL for 15 min.
3.1.3. The amount of NO2 vapor
collected in the first and second sections of the tubes was
measured. The collection efficiency was calculated by dividing the
amount collected in the first
Results: Results are reported in Table 3. Collection efficiency was adequate at two times the Transitional PEL with an average recovery of 97%.
3.2. Breakthrough
Procedure: Samples were generated at a concentration greater than the evaluation levels to determine the extent of NO2 breakthrough from the first solid sorbent tube into a second tube. The calculated breakthrough should be less than 5%.
- 3.2.1. Four sampling tubes (SKC lot no. 374) were connected to
backup tubes and then to sampling pumps. Air samples were collected
for 15 min at a concentration of approximately 4 times the
Transitional PEL. The generation system was set at 30% RH and 25 °C.
The low humidity level was used as a "worst case" test since the
presence of water is necessary for the conversion reaction of
NO2 to
3.2.2. Breakthrough was assessed by analyzing both tubes and
dividing the amount collected in the second
Results: The amount of breakthrough is shown in Table 3. Breakthrough studies indicate the sorbent tube capacity for NO2 is adequate for air concentrations at least to 21 ppm (using air volumes and flow rates described). Small amounts of NO2 were detected on the backup tubes during both collection efficiency and breakthrough studies. This could be from contamination rather than actual breakthrough. Although sample results are blank corrected, blank readings can be variable (see Section 7 and Table 7 for further information regarding blanks). Regardless of blank contamination or breakthrough, the breakthrough recoveries for both studies are less than 5% and are considered acceptable.
4. Storage Stability
A study was conducted to determine any effects on storage of
- 4.1. The determination was performed using SKC lot no. 306 tubes.
4.2. Twenty-four samples were generated at the OSHA Transitional PEL as described in the Introduction.
4.3. These samples were stored at 20 to 25 °C and were placed laboratory bench for the duration of the storage period.
4.4. Six samples were analyzed after 1, 5, 15, and 29 days.
Results: The results of the storage stability study are
shown in Table
4. Collected samples are stable at room temperature. The mean of
samples analyzed after 29 days was within ±5% of the mean of samples
analyzed after one day. Samples may be stored in normal environmental
conditions found in a laboratory setting for a period of 29 days after
sampling without producing a significant change in results. Procedure: A study was conducted to evaluate any effects
on recovery when sampling at different humidities. A contaminant flow
conditioned at different relative humidities and a constant temperature of
25 °C was generated using the system described in the Introduction.
Relative humidities of 30, 50, and 80% were used. SKC lot no. 374 tubes
were used and six samples were generated at each humidity level.
Results: Results are shown in Table
5. Data from sampling at different humidities displayed no apparent
effect on sampling efficiency. As shown in Table
5, an analysis of variance (F test) was performed on the data to
determine if any significant difference existed in different humidity
group results. The average recovery across the three different humidity
levels was also considered. The calculated F value is below the critical
value and a significant effect from humidity does not appear to exist.
Evidence of a slight increase in average recovery is apparent with an
increase in humidity. However, the increase is within the variability of
the method and also does not appear as significant. Therefore, the
humidity study did not reveal a significant difference in recoveries or
variance when sampling at 30, 50, and 80% RH (25 °C).
6. Comparison of Analytical Methods
The IC method was compared to a reference method to determine if any
significant disagreement existed between the 2 methods. The previous
analytical method, the differential pulse polarographic (DPP) procedure
(11.9.),
was used as the reference analytical method. Procedure: Eighteen samples were generated and analyzed
by IC. Since both analytical procedures use the same desorbing solution
[(1.5% triethanolamine (TEA)], an aliquot was taken from each sample and
analyzed by the polarographic method.
Results: A linear regression comparison of the two
methods is shown in Figure
2 (the dotted line shown in Figure 2 represents ideal agreement
between the two methods. The solid line represents the observed
agreement). Results of the comparison between the IC and DPP method are
also shown in Table
6. The comparison of the DPP and IC analytical methods show excellent
correlation and agreement. The correlation coefficient (r) of 0.99 and a
slope value of 1.0194 ± 0.0295 are very close to ideal values. An r and
slope value equal to 1 would indicate ideal correlation and agreement
between the two analytical methods. Over the concentration range tested
the IC method results show an increase of 1.9% when compared to
polarographic method results. The slightly higher recoveries of the IC
procedure indicate that some of the bias noted (Section 2) can be
attributed to the analytical portion of the method. The background levels
inherent in the treated sorbent and erratic blank readings probably
contribute to the positive bias also.
7. Analytical Detection Limits
Procedure: Qualitative and quantitative detection limits
were determined by analyzing low concentration samples and blanks. The
samples were prepared by spiking solutions containing 3 mL of 1.5% TEA
with sodium nitrite solutions. The spiking was performed using a
calibrated micropipette. Samples and blanks were analyzed using a 50 µL
sample injection loop and a conductivity cell sensitivity range setting of
3 microsiemens.
7.2. Quantitative detection limit: The International Union of Pure
and Applied Chemistry (IUPAC) detection limit equation (11.11.)
was used to calculate the detection limit. Results: The results are listed in Table
7 and graphically displayed in Figure
3. The qualitative detection limit is 0.07 ppm
NO2. The quantitative detection limit is 0.19
ppm NO2. A 50 µL sample injection loop was used
for all analyses in this evaluation. If necessary, a larger sample loop
can be used to achieve a lower limit of detection. In the past, blank
contamination was a serious problem and consequently caused high detection
limits; blank levels were occasionally 0.5 to 1 times the Transitional PEL
when using a 0.05 L/min flow rate for calculations. Soluble chloride salts
can also elevate the detection limit. If the amount of chloride in the
sample is large (>5 µg/mL), the nitrite ion appears as a shoulder on
the chloride peak during IC analysis. Using the data reduction system
described in Section 2 of the method (11.1.), the
proposed factor for the conversion of NO2 gas to
the nitrite ion is concentration dependent. If the reaction is
stoichiometric, a C.F. of 0.5 would be seen experimentally. In practice,
however, this is not the case. For concentrations below 10 ppm, the
average C.F. is 0.6 to 0.7 as reported by. Morgan et. al. (11.12.),
in a previous study (11.9.),
and by numerous others
8.2. The generation system was set at 50% RH and 25 °C.
8.3. Samples were taken using impingers containing 1.5% TEA
solutions for variable time periods at different concentration ranges.
These TEA solutions were used in an attempt to avoid any extraneous
background contribution from solid sorbent desorption or intrinsic
contamination from the tubes. Samples were taken at a flow rate of
0.025 L/min to assure complete oxidation of the NO and to provide
sufficient residence time of NO2 in the TEA
solutions. Results: The results for C.F. calculations from 10 to 200
ppm are listed in Table
8. Data in Table 8 show the conversion factors for
NO2 concentrations from 10 to 200 ppm. The
conversion factor for the 10 to 100 ppm concentration range averaged 0.50;
at about 200 ppm the factor was 0.37. Further work may be necessary to
determine why the factor decreased at the 200 ppm level. Another study
indicated no breakthrough of NO at this concentration (11.14.).
Previous sample results and the toxicology of
NO2 indicate a 200 ppm
NO2 sample collected in an industrial setting is
unlikely. A correction factor and further work at this concentration level
was not pursued for these reasons. The conversion factor is further
discussed in reference 11.14.
9. Sampling and Analysis - Supelco Tubes
A preliminary evaluation of the combination tube manufactured by
Supelco was conducted using the same conditions and equipment mentioned in
the Introduction. Samples were collected using the procedure mentioned in
Section 2. Results are listed in Table 9. This data indicates a sample
loss of approximately 30% when sampling at approximately 0.2 L/min. The
loss could be associated with a difference in mesh size (Supelco tubes
contain a smaller mesh molecular sieve than SKC tubes), flow rate
differences or a poorly prepared lot. The original methodology for
sampling NO2/NO with this type of tube specified
a flow rate of less than or equal to 0.05 L/min. The
10. Discussion
Two different lots of SKC tubes were used for the evaluation. The
combination tube consisting of all three sections in a single tube (lot
no. 306) was commercially available at the beginning of the evaluation.
This tube was used for the sampling and analysis, collection efficiency,
and storage stability experiments. Design changes were instituted and a
three tube collection device was produced to offer greater convenience
when sampling NO2 or both NO and
NO2 simultaneously. The The data generated during the evaluation of the method indicates an
acceptable alternative to the polarographic method. The ion
chromatographic method offers an accurate and precise determination of
NO2 exposures. A
11. References
11.2. Saltzman, B.E.: Colorimetric
Microdetermination of Nitrogen Dioxide in the Atmosphere. Anal.
Chem. 26:1949 (1954).
11.3. Gold, A.: Stoichiometry of Nitrogen
Dioxide Determination in Triethanolamine Trapping Solution. Anal.
Chem. 11.4. Blacker, J.H.: Triethanolamine for
Collecting Nitrogen Dioxide in the TLV Range. Am. Ind. Hyg. Assoc.
J. 34:390 (1973).
11.5. Vinjamoori, D.V. and Chaur-Sun Ling:
Personal Monitoring Method for Nitrogen Dioxide and Sulfur Dioxide
with Solid Sorbent Sampling and Ion Chromatographic Determination.
Anal. Chem. 11.6. Occupational Safety and Health
Administration Analytical Laboratory: Precision and Accuracy Data
Protocol for Laboratory Validations. In OSHA Analytical Methods
Manual. Cincinnati, OH: American Conference of Governmental
Industrial Hygienists (Pub. No. ISBN: 11.7. National Institute for Occupational
Safety and Health: Documentation of the NIOSH Validation
Tests by D. Taylor, (DHEW/NIOSH Pub. No. 11.8. Mandel, J. In Treatise on
Analytic Chemistry. 2nd ed. Kolthoff, I.M. and Elving, P.J., ed.
New York: John Wiley and Sons, Inc., 1978. p 282.
11.9. Occupational Safety and Health
Administration Analytical Laboratory: OSHA Analytical Methods
Manual 11.10. Dixon, W.J. and F.J. Massey, Jr.:
Introduction to Statistical Analysis. 2nd ed. New York:
11.11. Analytical Methods Committee:
Recommendations for the Definition, Estimation and Use of the
Detection Limit. Analyst 11.12. Morgan, G.B., C. Golden, and E.C.
Tabor: "New and Improved Procedures for Gas Sampling and Analysis
in the National Air Sampling Network" Paper presented at the 59th
Annual Meeting of the Air Pollution Control Association, San
Francisco, CA, 1966.
11.13. National Institute for Occupational
Safety and Health: NIOSH Manual of Analytical Methods by D.
Taylor, (DHEW/NIOSH Pub. No. 11.14. Occupational Safety and Health
Administration Technical Center: Nitric Oxide Backup Data
Report (ID-190) by J.C. Ku. Salt Lake City, UT. Revised 1991.
A block diagram of the major components of the dynamic generation
system is shown below. The system consists of four essential elements, a
flow, temperature and humidity control system, a nitrogen dioxide vapor
generating system, a mixing chamber and an active sampling manifold.
7.1. Qualitative detection limit: The Rank Sum Test (11.10.)
was used for the determination of the qualitative detection limit of
the IC analysis of NO2 (as nitrite).
8.1. A cylinder of NO in nitrogen (Air Products Co., 1.05% NO) was
used as the contaminant source. The rapid depletion of the
NO2 permeation tubes precluded their use for
this experiment. The same generation system shown in Figure 1 was used
with the gas cylinder replacing the permeation tubes as the
contaminant source. The NO2 was produced by
flowing a diluted NO mixture through oxidizer sections, which
converted the NO to NO2 before collection.
11.1. Occupational Safety and Health
Administration Technical Center: Nitrogen Dioxide in Workplace
Atmospheres (Ion Chromatography), by J.C. Ku
Analysis*
Nitrogen Dioxide
Level**
------0.5 × PEL-----
------ 1 × PEL-----
------ 2 × PEL-----
µg
takenµg
foundDE
µg
takenµg
foundDE
µg
takenµg
foundDE
12.35
15.48
12.59
15.72
13.26
12.28
12.08
14.9712.50
16.35
13.11
16.80
13.90
13.10
12.55
16.461.01
1.06
1.04
1.07
1.05
1.07
1.04
1.10
23.78
29.82
27.44
25.25
24.88
31.1024.38
24.12
29.31
27.00
26.33
33.361.03
***
1.07
1.07
1.06
1.07
58.36
53.06
52.14
65.68
57.08
52.3360.68
54.42
54.95
68.36
61.73
54.911.04
1.03
1.05
1.04
1.08
1.05
n
Mean
Std Dev
CV1
8
1.06
0.027
0.025
5
1.06
0.017
0.016
6
1.05
0.017
0.016
CV1 (pooled) = 0.021
Ave.
DE = 1.06
DE = Desorption efficiency
*
SKC tubes, lot no. 374, were used
**
Transitional PEL of 5 ppm NO2 was
used
*** Excluded from statistical analysis as an
outlier
Sampling and Analysis*
Nitrogen Dioxide
Test Level**
Found
µgAir Vol
(L)Found
ppmTaken
ppmRecovery
(in %)
0.5 × PEL
14.46
12.32
10.59
14.37
16.03
15.022.59
2.23
1.94
2.62
2.81
2.672.97
2.94
2.90
2.92
3.03
2.992.64
2.64
2.64
2.64
2.64
2.64113
111
110
111
115
113
n
Mean
Std
Dev
CV2 6
112
1.8
0.016
1 × PEL
28.77
23.61
21.01
27.59
28.24
29.172.59
2.23
1.94
2.62
2.81
2.675.90
5.63
5.76
5.60
5.34
5.815.06
5.06
5.06
5.06
5.06
5.06117
111
114
111
106
115
n
Mean
Std
Dev
CV2 6
112
3.9
0.035
2 × PEL
56.83
46.99
38.21
53.06
55.53
54.392.59
2.23
1.94
2.62
2.81
2.6711.66
11.20
10.47
10.76
10.50
10.839.45
9.45
9.45
9.45
9.45
9.45123
119
111
114
111
115
n
Mean
Std
Dev
CV2 6
115
4.8
0.042
CV2
(pooled)
Ave. Recovery= 0.033
= 113%CVT
(pooled)
Overall Error= 0.034
= ±19.8%
* SKC tubes, lot no. 306, were used
**
Transitional PEL of 5 ppm NO2 was
used
Collection Efficiency
(25 °C and 50%
RH)
------ µg NO2 Found
-----------
Sample No.
First Section
Second Section
% Collection Efficiency
1
2
3
4
5
656.83
46.99
38.21
53.06
55.53
54.392.32
ND
ND
2.19
1.98
2.3096.1
100.0
100.0
96.0
96.6
95.9
Average
97.4
Note:
(1)
SKC tubes, lot no. 306, were used
(2)
Sampling rate
Sampling time=
=0.2 L/min
15 min
(3)
Concentration
=
approximately 2 times OSHA Transitional PEL
(4)
ND
=
None detectable < 0.24 µg
NO2¯
(3-mL sample
volume)
(25 °C and 30% RH)
------ µg NO2 Found
-----------
Sample No.
1st Tube
2nd Tube
% Breakthrough
1
2
3
4103.8
104.5
105.1
103.23.34
ND
3.31
ND3.1
0
3.1
0
Average
1.6
Note:
(1)
1st and 2nd tube
=
SKC tubes, lot no. 374, were used
(2)
Sampling rate
Sampling time=
=0.175 L/min
15 min
(3)
Generation concentration
=
21 ppm NO2
(4)
ND
=
None detectable < 0.24 µg
NO2¯
(3-mL sample volume)
Storage Stability Test*
Nitrogen
Dioxide
Storage Day
Found
µgAir Vol
(L)Found
ppmTaken
ppmRecovery
(%)
Day 1
28.77
23.61
21.01
27.59
28.24
29.172.59
2.23
1.94
2.62
2.81
2.675.90
5.63
5.76
5.60
5.34
5.815.06
5.06
5.06
5.06
5.06
5.06117
111
114
111
106
115
n
Mean
Std Dev
CV 6
112
3.9
0.035
Day 5
25.74
23.56
20.69
26.52
29.32
28.412.61
2.23
1.93
2.60
2.72
2.615.24
5.61
5.70
5.42
5.73
5.795.04
5.04
5.04
5.04
5.04
5.04104
111
113
108
114
115
n
Mean
Std Dev
CV 6
111
4.2
0.038
Day 15
24.56
22.64
20.56
28.27
29.50
28.692.61
2.23
1.93
2.60
2.72
2.615.00
5.40
5.66
5.78
5.76
5.845.04
5.04
5.04
5.04
5.04
5.04 99.2
107
112
115
114
116
n
Mean
Std Dev
CV 6
111
6.4
0.058
Day 29
27.34
23.95
23.66
27.58
28.41
31.922.61
2.23
1.93
2.60
2.72
2.615.57
5.71
6.52
5.64
5.55
6.505.04
5.04
5.04
5.04
5.04
5.04111
113
129
112
110
129
n
Mean
Std Dev
CV 6
117
9.2
0.079
* SKC tubes, lot no. 306 were
used
Relative Humidity Test (25 °C)*
Generated
NO2 Concentration = 2.64 ppm
RH, %
34
50
80
NO2 Found, ppm
2.74
2.73
2.65
3.11
2.73
2.772.79
2.94
2.90
2.92
3.03
2.992.88
2.81
2.79
2.91
3.10
2.86
n
Mean, ppm
Std Dev, ppm
CV
Recovery6
2.79
0.16
0.058
106%6
2.93
0.083
0.028
111%6
2.89
0.11
0.038
109%
F test results: Fcalc
= 2.078, Fcrit
= 6.36, p < 0.01
* SKC tubes, lot no. 374,
were used
Comparison of Methods*
[Ion Chromatographic (IC)
vs. Polarographic (DPP)]
0.5 × PEL**
-- ppm Found -- 1 × PEL**
-- ppm Found -- 2 × PEL**
-- ppm Found --
IC
DPP
RR
IC
DPP
RR
IC
DPP
RR
3.41
3.43
3.57
3.40
3.46
3.393.17
3.19
3.27
3.19
3.33
2.821.076
1.075
1.092
1.066
1.039
***5.93
6.21
6.21
5.95
5.88
6.155.55
6.03
5.91
5.61
5.58
6.001.068
1.030
1.051
1.061
1.054
1.02510.11
10.20
10.73
10.33
10.11
11.9610.11
10.39
10.23
10.09
10.03
10.491.000
0.982
1.049
1.024
1.008
1.140
n
Mean
Std Dev
CV5
1.070
0.020
0.0186
1.048
0.017
0.0166
1.034
0.057
0.055
* SKC tubes, lot no. 374, were used
**
Transitional PEL of 5 ppm NO2 was
used
*** Excluded from statistical analysis as an
outlier
RR = Relative ratio, IC Found (ppm)/DPP
Found (ppm)
Correlation coefficient (r)
= 0.9938
Slope (b)
= 1.0194
Intercept (a)
= 0.1587
Std dev of slope (Sb)
= 0.0295
Qualitative Detection Limit - Nitrogen
Dioxide
Rank Sum Test
For n(s) = n(b) =
10
-------------------------
NO2 -- (as nitrite)
---------------------------
Rank
0.08 µg/mL
Peak Area
0.16 µg/mL
Peak Area
0.32 µg/mL
Peak Area
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
200.50
0.57
0.70
0.72
0.83
1.03
1.05
1.13
1.13
1.16
1.86
1.89
1.89
1.89
1.93
1.99
2.10
2.10
2.16
2.18RBL
RBL
RBL
RBL
RBL
RBL
RBL
RBL
RBL
RBL
STD
STD
STD
STD
STD
STD
STD
STD
STD
STD0.50
0.57
0.70
0.72
0.83
1.03
1.05
1.13
1.13
1.16
2.14
2.29
2.41
2.46
2.58
2.77
2.79
2.83
2.90
2.93RBL
RBL
RBL
RBL
RBL
RBL
RBL
RBL
RBL
RBL
STD
STD
STD
STD
STD
STD
STD
STD
STD
STD0.50
0.57
0.70
0.72
0.83
1.03
1.05
1.13
1.13
1.16
4.02
4.15
4.33
4.49
4.61
4.64
4.67
4.78
4.81
4.96RBL
RBL
RBL
RBL
RBL
RBL
RBL
RBL
RBL
RBL
STD
STD
STD
STD
STD
STD
STD
STD
STD
STD
Rb =
C =55
99.9%55
99.9%55
99.9%
Qualitative detection limit for nitrogen dioxide
= 0.08 µg/mL or 0.24 µg
Note:
(1) RBL
= Reagent Blank
(2) STD
= Standard
(3) Peak Area
= measured peak
area/100,000
Quantitative Detection Limit - Nitrogen
Dioxide (as NO2¯)
Sample No.
Blank
Peak Area0.08 µg/mL
Peak Area0.16 µg/mL
Peak Area0.32 µg/mL
Peak Area
1
2
3
4
5
6
7
8
9
100.50
0.57
0.70
0.72
0.83
1.03
1.05
1.13
1.13
1.161.86
1.89
1.89
1.89
1.93
1.99
2.10
2.10
2.16
2.182.14
2.29
2.41
2.46
2.58
2.77
2.79
2.83
2.90
2.934.02
4.15
4.33
4.49
4.61
4.64
4.67
4.78
4.81
4.98
n
Mean
Std Dev
CV10
0.88
0.25
0.28210
2.00
0.12
0.06210
2.61
0.27
0.10510
4.55
0.30
0.067
Peak Area = measured peak area/100,000
The quantitative detection limit is calculated using the
equation:
Cld
= k(sd)/m
Cld
= 10(0.248)/10.83 = 0.23 µg/mL
Where:
Cld =
the smallest reliable detectable concentration an
analytical instrument can determine at a given confidence
level
k =
10, thus giving confidence that any detectable signal
will be greater than or equal to an average blank reading plus ten
times the standard deviation (area reading >
Blave + 10sd)
sd =
standard deviation of blank readings
m =
analytical sensitivity or slope as calculated by
linear regression
Quantitative detection limit =
0.23 µg/mL (as nitrite) or 0.69 µg
Nitrogen Dioxide Conversion factor
NO2 ppm
Samples
Std Dev
CV
Mean*
12.89
25.20
49.79
97.90
192.57 7
7
6
6
70.038
0.037
0.022
0.020
0.0250.074
0.070
0.043
0.044
0.0680.519
0.533
0.517
0.450
0.368
* Average conversion factor. This was calculated from
sample results and assumed a 100% recovery.
Sampling and Analysis (Supelco Tubes)*
Nitrogen
Dioxide
Test Level**
Found
µgAir Vol
(L)Found
ppmTaken
ppmStatistics
0.5 × PEL
5.80
6.25
7.71
8.52
5.06
8.71
2.19
1.90
1.71
2.24
2.40
2.561.41
1.75
2.40
2.02
1.12
1.812.62
2.62
2.62
2.62
2.62
2.62
n
Mean
Std Dev
CV 6
66.9%
17.0
0.26
1 × PEL
15.91
12.73
11.45
15.82
16.27
18.942.19
1.90
1.71
2.24
2.40
2.563.86
3.56
3.56
3.75
3.60
3.935.08
5.08
5.08
5.08
5.08
5.08
n
Mean
Std Dev
CV 6
73.0%
3.2%
0.043
2 × PEL
30.11
22.77
22.48
28.63
31.14
34.042.19
1.90
1.71
2.24
2.40
2.567.31
6.35
6.99
6.79
6.90
7.079.66
9.66
9.66
9.66
9.66
9.66
n
Mean
Std Dev
CV 6
71.4%
3.3%
0.047
* Supelco tubes, Lot No.
** Transitional PEL of 5 ppm
NO2 was used
CV(pooled) = 0.15
Ave. Recovery = 70.4%
Overall Error = ±59.6%
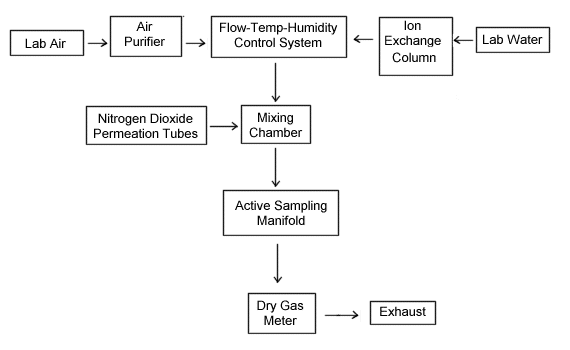
Figure 1
Linear Regression Comparison
Ion
Chromatographic vs. Polarographic Analysis of Nitrogen Dioxide
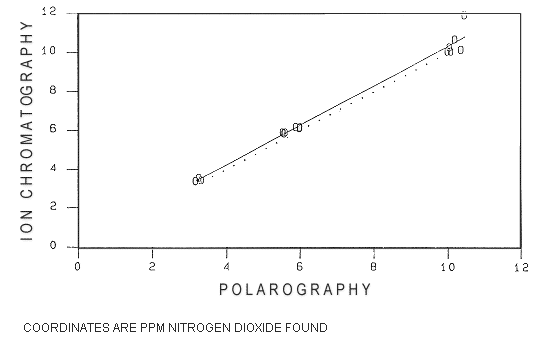
Dotted Line = Ideal Agreement
Between Methods
Solid Line
= Found Agreement Between Methods
Figure 2
Detection Limit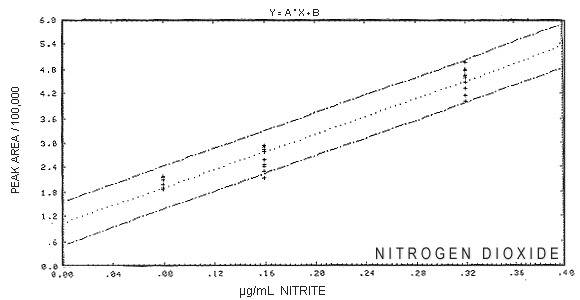
Figure 3