Introduction
The general procedure for
collection and analysis of air samples for phosphine is described in OSHA
Method No. ID-180 (11.1). Phosphine is collected on a solid sorbent
composed of beaded activated carbon impregnated with potassium hydroxide
(KOH). The sampling and analytical procedure is based on the following
proposed chemical reaction:
| 3 PH3 + 6 OH¯ + 5
O2 -------> 6 H2O +
HPO42¯ + 2
HPO32¯ |
For every 3 moles of phosphine, 1 mole of phosphate
(HPO42¯ ) and 2 moles of phosphite
(HPO32¯) are produced. The collected phosphine is
extracted from the carbon bead using 30% hydrogen peroxide
(H2O2) and analyzed as phosphite by ion
chromatography (IC). An additional analytical confirmation by IC for
phosphate was not performed. The KOH used for impregnating the sorbent
contained high background levels of phosphate.
This method has been
validated for a 36-L, 240-min sample based on a pump flow rate of
approximately 0.15 L/min. All solid sorbent tubes used during the
validation contained single sections consisting of approximately 1.5 g of
treated carbon. The majority of tubes used were prepared in-house;
exceptions are noted where commercially prepared tubes were used. Two
different size tubes (9-mm and 5-mm o.d.) were obtained from a commercial
source (SKC Inc., Eighty Four, PA). A significant difference in recoveries
between the different size tubes was not noted during side-by-side
testing.
A cylinder of phosphine (1.02% phosphine in nitrogen,
certified, Air Products Co.) was used for generating test atmospheres. An
evaluation (11.2) of the cylinder concentration using the phosphomolybdate
method of analysis (11.3) indicated the manufacturer's stated
concentration was accurate. The stated concentration was used for all
calculations of theoretical (taken) concentrations. A dynamic generation
system (described in Section 2 and Figure 1) was used for all experiments
except for the detection limit and field evaluation tests. Detection limit
tests were performed using sodium phosphite spikes. Samples for all
experiments were analyzed IC (11.1).
The method validation
consisted of the following experiments and summaries:
| 1. |
|
An analysis of 22 spiked samples to
determine desorption efficiency and analytical precision and
accuracy. |
| 2. |
|
An analysis of 22 samples collected
from dynamically generated test atmospheres to determine overall
precision and accuracy. |
| 3. |
|
A determination of the sampling media
collection efficiency. |
| 4. |
|
Determinations of breakthrough when
sampling time or concentration is increased or when sampling in low
humidity environments. |
| 5. |
|
An evaluation of the room temperature
storage stability of 38 samples taken at the OSHA Permissible
Exposure Limit (PEL) of 0.3 ppm. An additional evaluation of the
effects of refrigeration on storage stability. |
| 6. |
|
A determination of any significant
change in recovery when sampling at different humidities. |
| 7. |
|
A study of the shelf-life of stored
sorbent tubes. |
| 8. |
|
A determination of the qualitative and
quantitative detection limits. |
| 9. |
|
A field evaluation of sampling media
at a grain processing mill. |
| 10. |
|
An assessment of the method and
summary. |
An Addendum
describing a comparison of different lots of carbon bead, and samples
generated at the STEL are included at the end of this backup report. The
study of different lots was conducted when it was discovered that some
lots of the carbon bead were less efficient at collecting phosphine than
previously expected.
Results were calculated using
concentration-response curves and were statistically examined for outliers
and homogeneity of variance. Possible outliers were determined using the
Treatment of Outliers test (11.4). Homogeneity of variance was determined
using the Bartlett's test (11.5).
1. Analysis
Twenty-two
samples were prepared by adding known amounts of phosphine to treated
solid sorbent tubes to determine desorption efficiency (DE) and recoveries
for the analytical portion of the method. An active method of spiking was
used with low flow (0.01 to 0.02 L/min) sampling pumps to determine the
amount of gas collected and not necessarily the sampling capability at the
low flow rate.
1.1 Procedure: Sampling tubes containing
treated carbon bead were spiked by a procedure similar to that described
in reference 11.6. The phosphine source mentioned in the Introduction
was diluted to approximately 50 ppm using the system shown in Figure 1.
Calibrated low flow-rate pumps were connected to the sampling manifold
and were used to deliver the spikes for measured time periods. Air used
to dilute the phosphine source was tempered to 50% RH and 25°C. Pumps
used for this experiment were Model No. 222-3-12 (SKC, Eighty Four, PA)
and were calibrated to collect samples at 0.010 to 0.020 L/min. Spikes
were approximately 6, 12, and 24 µg phosphine. These levels are
approximately 0.5, 1, and 2 times the PEL for a 36-L air
sample.
1.2 Results: Recoveries are presented in Table 1. For the
analytical section of the method, the overall DE was 96.8%, and the
analytical precision (CV1 pooled) was 0.030. One result was
omitted as an outlier (1 × PEL group). Results for the three test levels
passed the Bartlett's test and were pooled. 2. Sampling and Analysis
To determine the precision
and accuracy of the method, known generated samples were prepared and
analyzed.
2.1 Procedure:
2.1.1 The phosphine gas source
mentioned in the Introduction was used to generate test atmospheres of
phosphine. This source was diluted with filtered, humidified air using
the system shown in Figure 1.
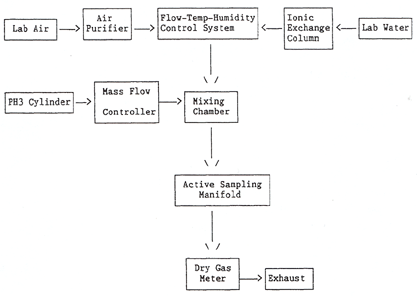
2.1.2 Dynamic generation system -
A Miller-Nelson Research Inc. (Model HCS-301) flow, temperature and
humidity control system was used for air flow control and
conditioning. All generation system fittings and connections were
Teflon. A glass mixing chamber was used to mix the tempered, filtered
air with the contaminant gas. The system was set to generate test
atmospheres at 50% RH and 25 °C.
2.1.3 The phosphine and
diluent air flow rates were adjusted using mass flow controllers. Flow
rates were also measured using a dry test meter (diluent air) and a
soap bubble flow meter (phosphine gas).
2.1.4 Samples were
taken from the sampling manifold using constant flow pumps. Calibrated
Du Pont P125 pumps were used. Pump flow rates were approximately 0.12
to 0.15 L/min and sampling times ranged from 240 to 360 min. Sample
concentrations were approximately 0.5, 1, and 2 times the OSHA PEL for
30- to 50-L air samples. 2.2
Results: Results are shown in Table 2. The precision and accuracy data,
based on NIOSH statistical protocol (11.5), is presented in Tables 1 and
2. The generated sample (Sampling and Analysis) results passed the
Bartlett's test and were pooled. The pooled coefficients of variation
are as follows:
| CV1(pooled) = 0.030 |
Cv2(pooled) = 0.039 |
CVT(pooled) =
0.041 |
There was
insignificant overall bias and the overall error was ±8.3%. Overall
error (11.7) was calculated using the equation: Overall error % =
±(|mean bias| + 2CVT ) × 100 (95% confidence)
3. Collection Efficiency
Procedure: Collection efficiency was determined for
in-house and commercially prepared tubes.
3.1 In-house Tubes
The collection
efficiency at the upper validation limit was determined using double
sampling tubes. Two sampling tubes were connected in series. These tubes
were prepared at the OSHA laboratory. Eight double tubes were connected
to the sampling manifold to collect samples at approximately 2 times the
OSHA PEL for 210 min (50% RH and 25°C). Pump flow rates were 0.12 to
0.15 L/min.
3.2 Commercial Tubes
Six double tubes were
also used to collect samples at 0.67 ppm for 240 min. Pump flow rates,
humidity and temperature were the same as mentioned in Section 3.1.
Tubes prepared by SKC Inc. (Eighty Four, PA.) were used.
3.3 The
amount of phosphine vapor collected in the first and second tubes was
determined. The collection efficiency was calculated by dividing the
amount collected in the first tube by the total amount of phosphine
collected in the first and second tube. Results: Results shown in Table 3
indicate a collection efficiency of 100.0% when sampling at approximately
2 times the OSHA PEL for 210 or 240 min. No breakthrough was evident
during the collection efficiency study.
4.
Breakthrough
Three different breakthrough experiments were
conducted to assess potential breakthrough:
Increased sampling time
Increased
concentration
Sampling in low humidity environments
4.1
Increased Sampling Time - Procedure: A preliminary study using solid
sorbent tubes and phosphine detector tubes (Model No. CH31101, Dräger,
Pittsburgh, PA) as colorimetric, qualitative indicators of breakthrough
was conducted. The Dräger tubes were chosen because of the very stable,
reproducible indication, large size, loose packing, and small pressure
drop when attached to a sampling pump. Two lots of Dräger detector tubes
had been previously tested at low concentration ranges when using
detector tube pumps (11.2). Additional testing was conducted with
continual flow pumps (DuPont P125) to assess the possibility of using
these detector tubes for identification of breakthrough. These tubes
were found capable of producing a noticeable colorimetric indication
after sampling 0.3 ppm phosphine for 15 min at a flow rate of 0.1 L/min
(approximately 0.6 µg phosphine). The detector tubes were attached
between the sampling tubes and pumps. Six samples were taken for 360 min
at 0.6 ppm and a flow rate of 0.12 to 0.15 L/min (50% RH and
25°C).
4.2 Increased Concentration - Procedure: Two sampling
tubes containing treated carbon bead were attached to each other and
five of these double tube samples were taken to determine breakthrough
at a concentration level of 1.9 ppm phosphine. Commercially prepared
(SKC Inc.) tubes were used for this experiment.
4.2.1 Samples were collected at a flow
rate of approximately 0.15 L/min, a concentration of 1.9 ppm
phosphine, 25°C and 50% RH. Samples were taken for 240
min.
4.2.2 Breakthrough was assessed by analyzing both tubes
and dividing the amount collected in the second solid-sorbent tube by
the total amount collected in both tubes. 4.3 Decreased Humidity - Procedure: An experiment was
conducted to determine if breakthrough exists at 30% RH. Sampling at
this humidity had given lower recoveries than expected (See Section 6.
for further details). This experiment should establish whether these low
recoveries are due to breakthrough or an incomplete or unanticipated
reaction of phosphine with the treated sorbent. To determine
breakthrough, sampling tubes, detector tubes, and pumps were connected
together as mentioned in Section 4.1. Samples were taken for 90 min at
flow rates of 0.115 to 0.135 L/min. The generation system parameters
were 1 ppm phosphine, 25°C, 30% RH. Results:
Detector tube color
indications were not observed after increasing the sampling time 360 min
at 2 times the PEL. As shown in Table 4, increasing the sampling
concentration to 1.9 ppm (240-min sampling time) produced breakthrough;
however, the amount was less than 5%. When sampling without a humidifier
at 30% RH, breakthrough was evident after a 6-µg sample load (Table 4).
Humidifiers are necessary at low RH (<40%) to prevent premature
breakthrough.
5. Storage Stability
A study was conducted to
assess the stability of phosphine collected on the treated solid
sorbent.
5.1 Procedure - storage assessment at
room temperatures of 20 to 25°C.
5.1.1 Thirty-eight samples were
collected using the generation system previously described in Section
2. Samples were collected at the PEL, 50% RH, and 25°C.
5.1.2
Samples were stored at room temperature (20 to 25°C) on a lab
bench.
5.1.3 Six to eight samples were analyzed after various
periods of storage (0, 5, 12, 18, and 32 days).
5.2 Procedure - refrigeration
assessment
5.2.1 Thirty-five samples were
collected in an effort to determine the effect of refrigeration on
sample storage stability. Twenty-four samples were collected at the
PEL and 11 at 2 times the PEL. The 11 samples were taken using
commercially prepared tubes while the other tubes were prepared
in-house.
5.2.2 Twelve samples taken at the PEL and four taken
at 2 times the PEL were analyzed immediately after
generation.
5.2.3 Nine samples were stored at room temperature
(20 to 25°C) and ten were refrigerated (7°C). Twelve samples were
analyzed after a 12- to 13-day storage period. The remaining seven
samples were analyzed after 30 days. 5.3 Results: Results of this stability study are shown in
Table 5 and Figure 2. The mean of samples (stored at 20 to 25°C)
analyzed was 81% of the known concentration after 12 days, 64% after 18
days, and 48% after a 32-day storage period. Results indicate samples
may be stored at typical laboratory temperatures up to 12 days after
sampling. Sample refrigeration reduces sample loss. Samples refrigerated
for 13 days (7°C) showed no apparent loss while those refrigerated for
30 days displayed a slight loss in recovery. Samples should be
refrigerated whenever possible. Since it is difficult to control the
temperature of samples after field collection and during shipment,
samples should be analyzed within 12 days of collection regardless of
laboratory refrigeration. 6.
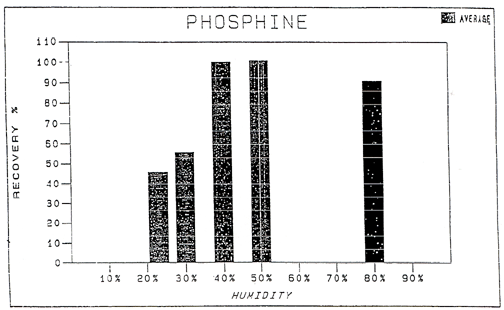
Humidity Study
A study was conducted to determine any significant
effect on recoveries when sampling at different humidities. Test
atmospheres were generated at 23, 30, 40, 50, and 80% RH. Samples were
taken for 360 min at flow rates of approximately 0.15 L/min. Results are
listed in Table 6 and displayed in Figure 3. As shown, samples collected
at relative humidities less than 40% result in unacceptably low phosphine
recoveries. This loss can be resolved by sampling with an in-line
humidifier.
The following techniques were used in an attempt to
humidify samples taken in areas having less than 40% RH:
6.1 Deionized water (0.75 mL) was added
to the end of a cellulose filter plug (Rainin Instrument Co., Woburn,
MA; part no. 23534/B) contained inside a glass tube. This tube was used
as a humidifying pre-tube. The 0.75 mL spike should not cause water
saturation to the treated sorbent and should provide continuous
humidification for up to 6 h of sampling at a flow rate of 0.15
L/min.
6.2 A 25-mL impinger containing approximately 5 mL of
deionized water was also used as a humidification device.
6.3
Procedure: Two different experiments were conducted. The first
experiment was performed at the PEL while the second was performed at a
higher concentration (1.042 ppm). The second experiment was performed to
evaluate the potential for humidifier failure or breakthrough at higher
concentration levels. Sampling rates for both experiments were 0.11 to
0.15 L/min. Samples were taken for 300 min during the first experiment
and 120 min for the high concentration test.
6.4 An additional
test was conducted to assess any change in recovery if the humidifier is
used at high humidity levels. Three samples with an in-line humidifier
and two samples without the humidifier were taken at about 2 times the
PEL. Sampling conditions were 25°C and 80% RH. Results: Results for either
humidifier are shown in Table 6. As shown, acceptable recoveries are noted
when the relative humidity is equal to or greater than 40% (25°C). The
results demonstrate the recovery was approximately 55% at 30% RH, and a
46% net recovery was noted at 23% RH. It is, therefore, concluded that the
relative humidities below 40% definitely result in low phosphine
recoveries. Results using a humidifier (Table 6) show acceptable
recoveries when sampling in low humidity environments; therefore, in-line
humidifiers are necessary for accurate assessments of phosphine when
sampling in low humidities (<40% RH).
The collection of samples
with an in-line humidifier at 80% RH was compared to samples taken without
a humidifier. Both approaches gave comparable recoveries. Average
recoveries were 107.6% and 112.5% for samples collected at 80% RH with a
humidifier and for samples collected without one, respectively. For added
convenience, humidity levels do not have to be determined if a humidifier
is used.
7. Shelf Life Study
A shelf life study of the
treated sampling tubes was conducted.
Procedure: Sampling tubes were prepared in-house and
sealed with plastic caps. After 68 days, the tubes were used to collect
samples from the generation system previously mentioned in Section 2.
Commercially prepared tubes were also set aside and used after a period of
6 months. These tubes were then used for the 0 day storage test (Table 5 -
Refrigeration vs. Room Temperature - 2 × PEL data).
Results: Commercially prepared tubes stored for 6 months
and then used to collect samples gave an average recovery of 113.7%.
Sealed tubes can be stored at typical laboratory temperatures (20 to 25°C)
for at least 6 months and then used for sampling.
8. Detection
Limits
Procedure: Detection limits were
determined by statistically examining the analytical results of spiked
samples and blanks. Low concentration samples were prepared by spiking
solutions of deionized water with sodium phosphite. Concentrations of
0.14, 0.28, and 0.57 µg/mL phosphite were used. Analysis of detection
limit samples was performed with a full scale detector output setting of 3
microsiemens. A 50-µL sample loop was used for all injections.
8.1 Qualitative Detection Limit - The
Rank Sum Test (11.8) was used for the determination of the qualitative
limit.
8.2 Quantitative Detection Limit - A modification or
derivation of the International Union of Pure and Applied Chemistry
(IUPAC) detection limit equation (11.9) was used to calculate the
quantitative limit. At the sensitivity level tested, blank readings and
the standard deviation of the blank were equal to zero. The lack of a
blank signal does not satisfy a strict interpretation of the IUPAC
detection limit when using the equation shown in Table 8. The
quantitative detection limit for this method is calculated using the
standard deviation of a standard below the range of the expected
detection limit as a substitute for the blank readings.
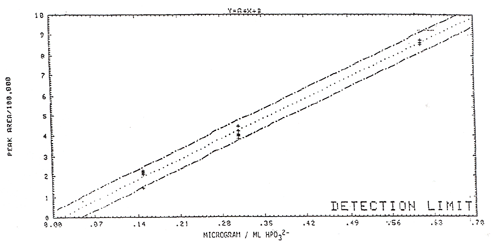
Results: The
results are listed in Table 8 and graphically displayed in Figure 4. The
qualitative detection limit is 0.14 µg/mL. The quantitative detection
limit is 0.23 µg/mL (both limits are calculated as HPO3²¯).
Using a 36-L air volume and a 5 mL sample volume, the qualitative limit is
0.009 ppm and the quantitative limit is 0.015 ppm phosphine (Table
8).
9. Field Evaluation
A field evaluation at a grain
processing mill was conducted using a number of different sampling
devices.
Procedure: A Teflon sampling
manifold was set-up with two exhaust pumps (DuPont P4000 pumps calibrated
at 3 L/min) at one end of the manifold. These pumps continually drew the
workplace air-phosphine mixture through the sampling manifold.
Side-by-side samples were taken from the manifold during a 3-h sampling
period in which Magtoxin (magnesium phosphide) was used to fumigate an
area in which grain is packaged and stored.
Mercuric
cyanide-impregnated silica gel tubes manufactured by SKC and Supelco as
well as commercially prepared carbon bead tubes were used. Carbon bead
samples were taken with two different size tubes. One tube had an outer
diameter of 5 mm (thin) while the other was 9 mm (large). Both tubes
contained approximately 1.5 g of treated sorbent. The thin tube was tested
since it could be used at higher humidity levels (without the humidifier)
and provide more convenience during use. The thin tube is easier to break
open and connect to a pump than the larger tube. The 9-mm o.d. tube is
used with the humidified filter tube since the dimensions of both tubes
are similar and are easily connected together with flexible
tubing.
Results: Results are listed in
Table 9. The field validation indicated good agreement between the
mercuric cyanide-treated silica gel tubes and the KOH-impregnated carbon
bead samples with in-line humidifiers. The results of the non-humidified
carbon bead samples are similar to the laboratory recoveries for low
humidity sampling (Table 6). A significant amount of breakthrough was
noted for the treated silica gel tubes at the apparent concentration of
1.4 to 1.5 ppm phosphine. The SKC tubes had less breakthrough than the
Supelco tubes at this concentration.
10. Summary
The results
indicate the method meets the NIOSH criteria for accuracy and precision
(11.5).
A side-by-side method comparison was not performed;
however, detector tube and mercuric cyanide-treated silica gel samples
were taken during the same period and under the same generation conditions
as the carbon bead tube. Recoveries for the silica gel samples were within
accepted limits (see reference 11.2 for further details).
Method
No. ID-180 has the disadvantage of low recoveries at low humidities if a
humidifier is not used. Unacceptable recoveries are also noted if samples
are stored at ambient temperatures longer than 12 days before analysis.
However, the overall method offers a simple, accurate and precise
assessment of phosphine concentrations if the appropriate steps are
taken:
1) When sampling in low humidity
environments, a humidifier is necessary. Humidifiers can be used
regardless of the humidity at the sampling site.
2) Analyses
should be performed within 12 days and samples should be refrigerated
during this time period. Experiments have been conducted after this backup report was
written. Please consult the Addendum at the end of this report for further
information.
11. References
11.1 Occupational Safety and Health
Administration Technical Center: Phosphine in
Workplace Atmospheres by J.C. Ku (OSHA-SLTC Method No. ID-180).
Salt Lake City, UT. Revised, 1991.
11.2 Occupational Safety and
Health Administration Analytical Laboratory: Phosphine Detector Tubes
(PE-4). Salt Lake City, UT. 1987.
11.3 National Institute for
Occupational Safety and Health: Manual of Analytical
Methods. 2nd. ed., Vol. 5 (Method No. S332) (DHEW/NIOSH Pub. No.
79-141). Cincinnati, OH: National Institute for Occupational Safety and
Health, 1979.
11.4 Mandel, J.: Accuracy and Precision, Evaluation
and Interpretation of Analytical Results, The Treatment of Outliers.
In Treatise On Analytical Chemistry, 2nd Ed.,
edited by I. M. Kolthoff and P. J. Elving. New York: John Wiley and
Sons, 1978. pp. 282-285.
11.5 National Institute for Occupational
Safety and Health: Documentation of the NIOSH
Validation Tests by D. Taylor, R. Kupel and J. Bryant (DHEW/NIOSH
Pub. No. 77-185). Cincinnati, OH: National Institute for Occupational
Safety and Health, 1977.
11.6 National Institute for Occupational
Safety and Health: Backup Data Report No. S332 for
Phosphine, Attachment A. Cincinnati, OH: National Institute for
Occupational Safety and Health, 1977 (unpublished).
11.7
Occupational Safety and Health Administration Analytical Laboratory:
Precision and Accuracy Data Protocol for Laboratory Validations. In
OSHA Analytical Methods Manual. Cincinnati,
OH: American Conference of Governmental Industrial Hygienists (Pub. No.
ISBN: 0-936712-66-X), 1985.
11.8 Dixon, W.J. and F.J. Massey,
Jr.: Introduction to Statistical Analysis. 2nd ed. New York: McGraw-Hill
Book Co., Inc., 1957. pp. 289-292, 445-449.
11.9 Long, G.L. and
J.D. Windfordner: Limit of Detection, A Closer Look at the IUPAC
Definition. Anal. Chem. 55: 712a-724a
(1983).
Table 1
Analysis
| Level* |
0.5 × PEL
|
1 × PEL
|
2 × PEL
|
|
µg
aken |
µg
found |
DE |
µg
taken |
µg
found |
DE |
µg
taken |
µg
found |
DE |
|
|
|
5.19 |
5.62 |
1.083 |
11.99 |
11.11 |
0.927 |
24.04 |
(LIA) |
- |
|
5.17 |
5.00 |
0.967 |
12.98 |
12.21 |
0.941 |
25.83 |
25.39 |
0.983 |
|
5.17 |
4.99 |
0.965 |
13.46 |
12.44 |
0.924 |
26.55 |
25.42 |
0.957 |
|
5.14 |
5.11 |
0.994 |
12.70 |
12.55 |
0.988 |
25.06 |
24.69 |
0.985 |
|
5.10 |
5.10 |
1.000 |
11.82 |
11.20 |
0.948 |
23.84 |
22.35 |
0.938 |
|
5.02 |
4.97 |
0.990 |
12.75 |
12.18 |
0.955 |
25.78 |
24.75 |
0.960 |
|
|
|
|
13.20 |
12.38 |
0.938 |
26.57 |
24.83 |
0.935 |
|
|
|
|
12.39 |
10.18 |
0.822** |
25.09 |
23.89 |
0.952 |
|
|
|
|
|
|
|
|
|
|
|
n |
|
6 |
|
|
7 |
|
|
7 |
|
Mean |
|
1.000 |
|
|
0.946 |
|
|
0.959 |
|
Std Dev |
0.043 |
|
|
0.022 |
|
|
0.020 |
|
CV1 |
|
0.043 |
|
|
0.023 |
|
|
0.021 |
CV1
(pooled) = 0.030
Ave. DE = 0.968 |
* Levels are approximate
** Excluded from statistical
analysis as an outlier
LIA = Lost In Analysis
DE = Desorption
Efficiency
Table 2
Sampling and Analysis
| Test Level |
--------- Found --------- |
|
Taken |
Recovery |
|
µg |
Air Vol (L) |
ppm |
|
ppm |
percent |
|
|
|
|
|
|
|
|
|
| 0.5 × PEL |
8.85 |
41.0 |
0.155 |
|
0.152 |
102.0 |
|
9.39 |
42.3 |
0.160 |
|
0.152 |
105.3 |
|
10.76 |
47.7 |
0.162 |
|
0.152 |
106.6 |
|
8.40 |
37.1 |
0.163 |
|
0.152 |
107.2 |
|
11.68 |
51.1 |
0.164 |
|
0.152 |
107.9 |
|
9.40 |
41.9 |
0.161 |
|
0.152 |
105.9 |
|
10.12 |
44.1 |
0.165 |
|
0.152 |
108.6 |
|
8.56 |
38.6 |
0.160 |
|
0.152 |
105.3 |
|
|
|
|
|
|
|
|
|
n |
8 |
|
|
|
|
|
Mean |
0.161 |
|
|
106.1 |
|
|
Std Dev |
0.003 |
|
|
|
|
|
CV2 |
0.019 |
|
|
|
| |
|
|
|
|
|
|
| 1 × PEL |
18.47 |
45.1 |
0.295 |
|
0.284 |
103.9 |
|
16.95 |
44.1 |
0.276 |
|
0.284 |
97.2 |
|
13.95 |
39.0 |
0.257 |
|
0.284 |
90.5 |
|
15.80 |
37.7 |
0.301 |
|
0.284 |
106.0 |
|
19.52 |
51.1 |
0.275 |
|
0.284 |
96.8 |
|
17.59 |
41.2 |
0.307 |
|
0.284 |
108.1 |
|
17.37 |
42.9 |
0.291 |
|
0.284 |
102.5 |
|
16.72 |
41.7 |
0.288 |
|
0.284 |
101.4 |
|
|
|
|
|
|
|
|
|
n |
8 |
|
|
|
|
|
Mean |
0.286 |
|
|
100.8 |
|
|
Std Dev |
0.016 |
|
|
|
|
|
CV2 |
0.057 |
|
|
|
|
|
|
|
|
|
|
| 2 × PEL |
28.35 |
35.1 |
0.581 |
|
0.666 |
87.2 |
|
23.55 |
28.6 |
0.592 |
|
0.666 |
88.9 |
|
27.22 |
31.8 |
0.616 |
|
0.666 |
92.5 |
|
26.21 |
30.2 |
0.624 |
|
0.666 |
93.7 |
|
27.80 |
32.9 |
0.608 |
|
0.666 |
91.3 |
|
26.57 |
30.6 |
0.624 |
|
0.666 |
93.7 |
|
|
|
|
|
|
|
|
|
n |
6 |
|
|
|
|
|
Mean |
0.608 |
|
|
91.2 |
|
|
Std Dev |
0.018 |
|
|
|
|
|
CV2 |
0.029 |
|
|
|
|
|
|
|
|
|
|
| * Excluded from
statistical analysis as an outlier |
|
CV2 (pooled) = 0.039 |
CVT (pooled) = 0.041 |
|
Bias =
0.1%
|
Overall
Error = ±8.3% |
Table 3
Collection Efficiency
50% RH,
25°C
|
-------- ppm Phosphine
-------- |
|
| Sample No. |
First Tube |
Second Tube |
% Collection
Efficiency |
|
| 1 |
0.49 |
ND |
100 |
| 2 |
0.55 |
ND |
100 |
| 3 |
0.58 |
ND |
100 |
| 4 |
0.58 |
ND |
100 |
| 5 |
0.51 |
ND |
100 |
| 6 |
0.54 |
ND |
100 |
| 7 |
0.52 |
ND |
100 |
| 8 |
0.57 |
ND |
100 |
| 9 |
0.58 |
ND |
100 |
| 10 |
0.59 |
ND |
100 |
| 11 |
0.62 |
ND |
100 |
| 12 |
0.62 |
ND |
100 |
| 13 |
0.61 |
ND |
100 |
| 14 |
0.62 |
ND |
100 |
Note:
(1) Samples 1 to 8 were
taken at 0.12 to 0.15 L/min flow rate for 210 min. These tubes were
prepared at the OSHA lab. Samples 9 to 14 are commercially prepared
tubes used to take samples for 240 min and at the same flow rates as the
in-house tubes.
(2) ND = None detectable < 0.015 ppm
phosphine
(3) Samples 1 to 8: Generation concentration = 0.6 ppm
phosphine
Samples 9 to 14: Generation concentration = 0.67 ppm
phosphine
Table 4
High Concentration
Breakthrough
50% RH, 25°C
|
------- µg
PH3 Found ------- |
|
| Sample No.
|
1st |
2nd |
% Breakthrough |
|
| 1 |
83.07 |
3.34 |
3.9 |
| 2 |
82.60 |
1.22 |
1.5 |
| 3 |
71.92 |
2.22 |
3.0 |
| 4 |
81.84 |
2.37 |
2.8 |
| 5 |
77.80 |
3.00 |
3.7 |
|
|
Ave: |
3.0 |
Note:
(1) 1st and 2nd =
Commercially prepared (SKC) sampling tubes
(2) Sample rate =
approximately 0.15 L/min flow rate for 240 min
(3) Generation
concentration 1.90 ppm phosphine
| ---------------------------------------------------------------------------------- |
Low Humidity Breakthrough
30% RH,
25°C
|
----- µg
PH3 Found ----- |
|
| Sample No. |
1st |
2nd* |
% Breakthrough |
|
| 1 |
5.69 |
3.90 |
40.6 |
| 2 |
5.45 |
2.50 |
31.5 |
| 3 |
5.62 |
3.20 |
36.3 |
| 4 |
5.66 |
1.88 |
24.9 |
| 5 |
5.61 |
3.13 |
35.8 |
| |
|
|
|
| n
|
5 |
5 |
|
| Mean |
5.61 |
2.92 |
33.8 |
| Std Dev |
0.093 |
0.76 |
|
| CV |
0.017 |
0.26 |
|
Note:
(1) 1st = Sampling
Tube; 2nd = Dräger phosphine detector tube
(2) Sampled at 0.115 to
0.135 L/min flow rate for 90 min
(3) Generation concentration
= 1 ppm phosphine
* Results obtained using calibrated detector
tubes. These results are approximate.
Table 5
Storage Stability Test
Ambient
(20 - 25°C) Storage
| (1 × PEL) |
--------- Found
----------- |
|
Taken |
% |
| Storage |
µg |
Air Vol (L) |
ppm |
|
ppm |
Recovery |
|
| Day 0 |
18.47 |
45.1 |
0.295 |
|
0.284 |
103.9 |
|
16.95 |
44.1 |
0.276 |
|
0.284 |
97.2 |
|
13.95 |
39.0 |
0.257 |
|
0.284 |
90.5 |
|
15.80 |
37.7 |
0.301 |
|
0.284 |
106.0 |
|
19.52 |
51.1 |
0.275 |
|
0.284 |
96.8 |
|
17.59 |
41.2 |
0.307 |
|
0.284 |
108.1 |
|
17.37 |
42.9 |
0.291 |
|
0.284 |
102.5 |
|
16.72 |
41.7 |
0.288 |
|
0.284 |
101.4 |
|
|
|
|
|
|
|
|
|
n |
8 |
|
|
|
|
|
Mean |
0.286 |
|
|
100.8 |
|
|
Std Dev |
0.016 |
|
|
|
|
|
CV |
0.057 |
|
|
|
|
|
|
|
|
|
|
| Day 5 |
16.70 |
44.0 |
0.273 |
|
0.284 |
96.1 |
|
16.85 |
45.2 |
0.268 |
|
0.284 |
94.4 |
|
16.15 |
42.8 |
0.271 |
|
0.284 |
95.4 |
|
12.08 |
39.4 |
0.220 |
|
0.284 |
* |
|
19.33 |
52.0 |
0.267 |
|
0.284 |
94.0 |
|
15.86 |
42.1 |
0.271 |
|
0.284 |
95.4 |
|
|
|
|
|
|
|
|
|
n |
5 |
|
|
|
|
|
Mean |
0.270 |
|
|
95.1 |
|
|
Std Dev |
0.002 |
|
|
|
|
|
CV |
0.009 |
|
|
|
|
|
|
|
|
|
|
| Day 12 |
12.90 |
41.4 |
0.224 |
|
0.300 |
74.7 |
|
14.19 |
42.4 |
0.241 |
|
0.300 |
80.3 |
|
16.63 |
47.8 |
0.250 |
|
0.300 |
83.3 |
|
13.27 |
37.4 |
0.255 |
|
0.300 |
85.0 |
|
16.99 |
50.3 |
0.243 |
|
0.300 |
81.0 |
|
13.54 |
41.0 |
0.237 |
|
0.300 |
79.0 |
|
15.28 |
43.2 |
0.254 |
|
0.300 |
84.1 |
|
16.60 |
37.9 |
0.315 |
|
0.300 |
* |
|
|
|
|
|
|
|
|
|
n |
7 |
|
|
|
|
|
Mean |
0.243 |
|
|
81.0 |
|
|
Std Dev |
0.011 |
|
|
|
|
|
CV |
0.045 |
|
|
|
* Excluded from statistical analysis
as outliers
Table 5 (Continued)
Storage Stability
Test
Ambient (20 - 25°C) Storage
| (1 × PEL) |
---------- Found
----------- |
|
Taken |
|
| Storage |
µg |
Air Vol (L) |
ppm |
|
ppm |
% Recovery |
|
| Day 18 |
12.55 |
44.2 |
0.204 |
|
0.280 |
72.9 |
|
11.88 |
43.7 |
0.195 |
|
0.280 |
69.6 |
|
10.96 |
41.7 |
0.189 |
|
0.280 |
67.5 |
|
9.58 |
38.6 |
0.178 |
|
0.280 |
63.6 |
|
11.53 |
51.1 |
0.162 |
|
0.280 |
57.9 |
|
9.87 |
41.5 |
0.171 |
|
0.280 |
61.1 |
|
9.08 |
43.4 |
0.150 |
|
0.280 |
53.6 |
|
11.55 |
42.8 |
0.194 |
|
0.280 |
69.3 |
|
|
|
|
|
|
|
|
|
n |
8 |
|
|
|
|
|
Mean |
0.180 |
|
|
64.4 |
|
|
Std Dev |
0.018 |
|
|
|
|
|
CV |
0.102 |
|
|
|
|
|
|
|
|
|
|
| Day 32 |
7.92 |
44.1 |
0.129 |
|
0.284 |
45.4 |
|
7.97 |
44.8 |
0.128 |
|
0.284 |
45.1 |
|
7.70 |
42.8 |
0.129 |
|
0.284 |
45.4 |
|
7.19 |
38.9 |
0.133 |
|
0.284 |
46.8 |
|
10.87 |
53.1 |
0.147 |
|
0.284 |
51.8 |
|
8.61 |
41.6 |
0.149 |
|
0.284 |
52.5 |
|
7.61 |
43.0 |
0.127 |
|
0.284 |
44.7 |
|
9.08 |
43.3 |
0.151 |
|
0.284 |
53.2 |
|
|
|
|
|
|
|
|
|
n |
8 |
|
|
|
|
|
Mean |
0.137 |
|
|
48.1 |
|
|
Std Dev |
0.010 |
|
|
|
|
|
CV |
0.077 |
|
|
|
Samples listed above were collected at
1 × PEL, 25°C and 50% RH.
Table 5 (Cont.)
Storage Stability
Test
Refrigeration (7°C) vs. Room Temperature (20 to
21°C)
|
---------- Found
------------- |
Taken |
|
|
ug |
Air Vol (L) |
ppm |
ppm |
% Recovery |
|
|
| Day 0 |
15.37 |
38.65 |
0.286 |
0.309 |
92.6 |
|
11.96 |
39.09 |
0.220 |
0.309 |
* |
| (1 × PEL) |
16.13 |
38.80 |
0.299 |
0.309 |
96.8 |
|
15.80 |
38.78 |
0.293 |
0.309 |
94.8 |
|
14.14 |
36.72 |
0.277 |
0.309 |
89.6 |
|
15.58 |
38.11 |
0.294 |
0.309 |
95.1 |
|
16.11 |
38.62 |
0.300 |
0.309 |
97.1 |
|
16.75 |
40.84 |
0.295 |
0.309 |
95.5 |
|
16.65 |
38.88 |
0.308 |
0.309 |
99.7 |
|
16.44 |
38.27 |
0.309 |
0.309 |
100.0 |
|
15.42 |
34.98 |
0.317 |
0.309 |
102.6 |
|
15.99 |
37.58 |
0.306 |
0.309 |
99.0 |
|
|
|
|
|
|
|
|
n |
11 |
|
|
|
|
Mean |
0.299 |
|
96.6 |
|
|
Std Dev |
0.011 |
|
|
|
|
CV |
0.038 |
|
|
| |
|
|
|
|
|
|
25.75 |
31.82 |
0.582 |
0.525 |
110.9 |
| (2 × PEL) |
26.53 |
31.62 |
0.603 |
0.525 |
114.9 |
|
25.58 |
30.88 |
0.596 |
0.525 |
113.5 |
|
26.54 |
31.37 |
0.608 |
0.525 |
115.8 |
|
|
|
|
|
|
|
|
n |
4 |
|
|
|
|
Mean |
0.597 |
|
113.7 |
| |
|
Std Dev |
0.011 |
|
|
|
|
CV |
0.019 |
|
|
*Tubing connecting the pump and
sampling tube disconnected during sampling. This result was not used
in statistical calculations.
Day 0 samples were desorbed and
analyzed immediately af ter collection. In-house prepared tubes were
used for 1 × PEL samples; commercial tubes were used for 2 × PEL
samples.
Table 5 (Cont.)
Storage Stability
Test
Refrigeration (7 °C) vs. Room Temperature (20 to
21°C)
|
|
|
|
|
|
----------
Found---------- |
Taken |
% |
|
|
µg |
Air Vol (L) |
ppm |
ppm |
Recovery |
|
|
|
| Day 13 |
16.37 |
39.11 |
0.301 |
0.309 |
97.4 |
Refrigerated |
|
15.77 |
36.95 |
0.307 |
0.309 |
99.4 |
|
| (1 × PEL) |
16.94 |
39.16 |
0.311 |
0.309 |
100.6 |
|
|
16.59 |
37.75 |
0.316 |
0.309 |
102.3 |
|
|
15.59 |
34.80 |
0.322 |
0.309 |
** |
|
|
15.93 |
37.31 |
0.307 |
0.309 |
99.4 |
|
|
|
|
|
|
|
|
|
|
n |
5 |
|
|
|
|
|
Mean |
0.308 |
|
99.8 |
|
|
|
Std Dev |
0.006 |
|
|
|
|
|
CV |
0.018 |
|
|
|
|
|
|
|
|
|
|
| Day 12 |
14.00 |
39.16 |
0.257 |
0.309 |
83.2 |
Room Temperature |
|
13.47 |
36.56 |
0.265 |
0.309 |
85.8 |
|
| (1 × PEL) |
14.61 |
39.79 |
0.264 |
0.309 |
85.4 |
|
|
13.55 |
37.20 |
0.262 |
0.309 |
84.8 |
|
|
11.97 |
34.17 |
0.252 |
0.309 |
81.6 |
|
|
13.78 |
37.25 |
0.266 |
0.309 |
86.1 |
|
| |
|
|
|
|
|
|
|
|
n |
6 |
|
|
|
|
|
Mean |
0.261 |
|
84.5 |
|
|
|
Std Dev |
0.005 |
|
|
|
|
|
CV |
0.021 |
|
|
|
|
|
|
|
|
|
|
| Day 30 |
19.81 |
30.99 |
0.460 |
0.523 |
88.0 |
Refrigerated |
|
22.48 |
31.35 |
0.516 |
0.523 |
98.7 |
|
| (2 × PEL) |
20.47 |
29.01 |
0.508 |
0.523 |
97.1 |
|
|
21.29 |
29.80 |
0.514 |
0.523 |
98.3 |
|
| |
|
|
|
|
|
|
|
|
n |
4 |
|
|
|
|
|
Mean |
0.500 |
|
95.5 |
|
|
|
Std Dev |
0.027 |
|
|
|
|
|
CV |
0.053 |
|
|
|
|
|
|
|
|
|
|
| Day 30 |
17.28 |
31.63 |
0.393 |
0.523 |
75.1 |
Room Temperature |
|
13.47 |
30.84 |
0.314 |
0.523 |
60.0 |
|
| (2 × PEL) |
15.94 |
29.65 |
0.387 |
0.523 |
74.0 |
|
|
|
|
|
|
|
|
|
|
n |
3 |
|
|
|
|
|
Mean |
0.365 |
|
69.7 |
|
|
|
Std Dev |
0.044 |
|
|
|
|
|
CV |
0.121 |
|
|
|
** Pump flow rate change was greater
than 10%. This result was not used.
Table 6
Humidity Experiments
(25°C)
| %RH |
23 |
30 |
40 |
50 |
80 |
|
|
| ppm PH3 Taken |
0.309 |
0.298 |
0.307 |
0.284 |
0.294 |
| |
|
|
|
|
|
| ppm PH3 Found |
0.148 |
0.169 |
0.302 |
0.295 |
0.257 |
|
0.134 |
0.181 |
0.306 |
0.276 |
0.262 |
|
0.133 |
0.175 |
0.315 |
0.257 |
0.272 |
|
0.151 |
0.192 |
0.308 |
0.301 |
0.271 |
|
0.159 |
0.105 |
0.301 |
0.275 |
0.267 |
|
0.135 |
0.195 |
|
0.307 |
0.274 |
|
|
0.198 |
|
0.291 |
0.158* |
|
|
0.105 |
|
0.288 |
0.268 |
|
|
|
|
|
|
| n |
6 |
8 |
5 |
8 |
7 |
| Mean, ppm |
0.143 |
0.165 |
0.306 |
0.286 |
0.267 |
| Std Dev, ppm |
0.011 |
0.038 |
0.006 |
0.016 |
0.006 |
| CV |
0.076 |
0.232 |
0.018 |
0.057 |
0.022 |
| Ave Recovery |
46.4% |
55.3% |
99.8% |
100.8% |
90.9% |
*
Excluded from statistical analysis as an outlier
Samples were taken
for 360 min at a flow rate of approximately 0.15 L/min.
Low
Concentration
Table 6 (Continued)
Humidifier vs. No
Humidifier
25°C & 30% RH
|
------- ppm
Phosphine Found ------ |
| Sample Type |
Humidifier |
No Humidifier |
|
| FP |
0.33 |
0.26 |
| FP |
0.44 |
0.22 |
| IMP |
0.34 |
0.28 |
| IMP |
0.34 |
0.28 |
| |
|
|
| n |
4 |
4 |
| Mean |
0.36 |
0.26 |
| Std Dev |
0.052 |
0.028 |
| CV |
0.143 |
0.109 |
| % Recovery |
90 |
65 |
| |
| Generation concentration
= 0.4 ppm phosphine |
| Sampling rate = 0.11 to
0.15 L/min |
| Sampling time = 300
min |
| ---------------------------------------------------------------------------------- |
High Concentration
|
---------- ppm
Phosphine Found ----------- |
|
---- Humidifier ---- |
No Humidifier |
| Sampling No. |
FP |
IMP |
|
|
|
| 1 |
0.9196 |
0.9608 |
0.3524 |
| 2 |
1.0271 |
0.9675 |
0.3413 |
| 3 |
1.0194 |
1.0221 |
0.2509 |
| 4 |
1.0314 |
0.9980 |
0.3460 |
| 5 |
1.0239 |
1.1107 |
0.3827 |
| 6 |
0.9186 |
1.0417 |
0.3191 |
| |
|
|
|
| n |
6 |
6 |
6 |
| Mean |
0.990 |
1.017 |
0.332 |
| Std Dev |
0.055 |
0.055 |
0.045 |
| CV |
0.056 |
0.055 |
0.135 |
| % Recovery |
95.0 |
97.6 |
31.9 |
| |
|
|
|
| Generation concentration
= 1.042 ppm phosphine |
| Sampling rate = 0.11 to
0.15 L/min, Sampling time = 120 min |
FP = cellulose plug was wetted with DI water prior to
sampling and used as pre-tube (Section 6.1).
IMP = an impinger
containing 5 mL DI water was placed in front of sampling tube (Section
6.2).
Table 7
Shelf Life
|
------ Found --------- |
Taken |
|
| Storage |
µg |
Air Vol (L) |
ppm |
ppm |
% Recovery |
|
| Day 0 |
18.63 |
42.9 |
0.312 |
0.297 |
105.1 |
|
16.38 |
37.4 |
0.315 |
0.297 |
106.1 |
|
18.76 |
41.4 |
0.326 |
0.297 |
110.0 |
|
15.09 |
37.6 |
0.289 |
0.297 |
97.3 |
|
|
|
|
|
|
|
|
n |
4 |
|
|
|
|
Mean |
0.310 |
|
104.5 |
|
|
Std Dev |
0.016 |
|
|
|
|
CV |
0.051 |
|
|
| |
|
|
|
|
|
| Day 68 |
17.33 |
42.0 |
0.297 |
0.297 |
100.0 |
|
20.88 |
48.0 |
0.313 |
0.297 |
105.4 |
|
22.09 |
50.3 |
0.316 |
0.297 |
106.4 |
|
19.15 |
43.2 |
0.319 |
0.297 |
107.4 |
| |
|
|
|
|
|
|
|
n |
4 |
|
|
|
|
Mean |
0.311 |
|
104.7 |
|
|
Std Dev |
0.010 |
|
|
|
|
CV |
0.032 |
|
|
Eight
blank sampling tubes were prepared in the laboratory. Four tubes were
used to take samples from the generation system (1× PEL, 25°C, 50% RH)
and then immediately analyzed (Day 0). The other 4 blank sampling tubes
were stored at 20 to 25°C for 68 days. Samples were then collected using
the same conditions as the Day 0 samples.
Commercial tubes were
stored for 6 months on a lab bench and then used for sampling. These
tubes were used to collect samples for the 0 Day Storage Stability
experiment (See Table 5 - Refrigerated vs. Room temperature, 0 Day
Storage, 2 × PEL for data). Four samples were collected at 2 times the
PEL, 25°C and 50% RH. Recoveries were 113.7% and the CV was
0.019.
Table 8
Qualitative Detection Limit
Rank
Sum Test
For n(s) = n (b) = 6
| |
-------- STD
Concentration (as HPO3²¯) ------- |
| |
0.14 µg/mL
|
0.28 µg/mL
|
0.57 µg/mL
|
| Rank |
PA |
SAM |
PA |
SAM |
PA |
SAM |
|
1
2
3
4
5
6
7
8
9
10
11
12 |
0.00
0.00
0.00
0.00
0.00
0.00
1.42
2.09
2.17
2.22
2.22
2.30 |
RBL
RBL
RBL
RBL
RBL
RBL
STD
STD
STD
STD
STD
STD |
0.00
0.00
0.00
0.00
0.00
0.00
3.87
4.00
4.06
4.06
4.23
4.47 |
RBL
RBL
RBL
RBL
RBL
RBL
STD
STD
STD
STD
STD
STD |
0.00
0.00
0.00
0.00
0.00
0.00
8.54
8.60
8.63
8.64
8.73
8.73 |
RBL
RBL
RBL
RBL
RBL
RBL
STD
STD
STD
STD
STD
STD |
| |
|
|
|
|
|
|
| Blank rank sum = |
21 |
|
21 |
|
21 |
|
| |
|
|
|
|
|
|
| Confidence level = |
99.9% |
|
99.9% |
|
99.9% |
Qualitative detection limit for phosphine = 0.14 µg/mL or
0.70 µg (5 mL sample volume). This corresponds to a 0.009 ppm phosphine
concentration for a 36-L air volume at 0.15 L/min sampling
rate.
Note: SAM denotes sample type:
(1) RBL = Reagent
Blank
(2) STD = Standard
PA = Integrated Peak Area
(HPO3²¯)/100,000
Table 8 (Continued
Quantitative Detection
Limit (IUPAC Method)
|
------- STD Concentration
(as HPO3²¯) -------- |
| Sample No. |
0.14 µg/mL
PA |
0.28 µg/mL
PA |
0.57 µg/mL
PA |
|
1
2
3
4
5
6
n
Mean
Std
Dev
CV |
1.42
2.09
2.17
2.30
2.22
2.22
6
2.07
0.33
0.16 |
4.47
4.06
4.06
4.23
4.00
3.87
6
4.12
0.21
0.05 |
8.63
8.73
8.64
8.54
8.73
8.60
6
8.65
0.07
0.01 |
| |
|
|
|
| PA =
Integrated Peak Area (
HPO3²¯)/100,000 |
The mean blank reading and standard deviation (Std dev) were
equal to zero.
Using the equation: Cld =
k(sd)/m
Where:
| Cld = |
the smallest reliable detectable
concentration an analytical instrument can determine at a given
confidence level. |
| k = |
10, thus giving greater than 99.9%
confidence that any detectable signal will be greater than or
equal to an average blank (or low standard) reading plus ten times
the standard deviation (area reading $ Blave + 10sd). |
| sd = |
standard deviation of low standard
readings. |
| m = |
analytical sensitivity or slope as
calculated by linear regression.
Cld =
10(0.33)/14.397 = 0.23 µg/mL
Quantitative detection limit =
0.23 µg/mL (as HPO3²¯) or 1.15 µg (5-mL
sample volume). This corresponds to 0.015 ppm phosphine for a 36-L
air volume at 0.15 L/min sampling rate. |
Table 9
Field Evaluation - Grain Processing
Mill
| Sampling conditions: |
26 to 30 % RH, 2°C |
| Sampling system: |
Samples were taken side-by-side
using a Teflon sampling manifold and two exhaust pumps. |
| Sampling time: |
3 h |
| Pumps: |
Du Pont P4000 |
| Sampling rates: |
0.11 to 0.14
L/min |
| |
|
|
|
| Tube Type |
n |
Average Results |
CV |
|
Supelco MC tube
SKC MC
tube
IBC large tube and humidifier
IBC large tube (no
humidifier)
IBC thin tube (no humidifier) |
3
3
3
3
3 |
1.41
ppm
1.51 ppm
1.43 ppm
0.63 ppm
0.56 ppm |
0.098
0.044
0.13
0.048
0.11 | Supelco MC and SKC MC tubes contain silica gel impregnated
with mercuric cyanide. Sampling and analysis for the silica gel tubes were
performed using the NIOSH phosphine method S332 (11.3) with some
modifications.
IBC = Impregnated Beaded Carbon. The thin tube
is approximately 170-mm long and 5-mm o.d. The large tube is approximately
110-cm long and 9-mm o.d. Both tubes contain 1.5 g of treated sorbent. All
IBC tubes were sampled and analyzed according to reference
11.1.
The humidifier is a cellulose filter plug saturated
with 0.75 mL deionized water.
| --------------------------------------------------------------------------------- | The Supelco MC and SKC MC tubes exhibited some breakthrough;
results listed above represent both A (front) and B (backup) sections. The
amount found in each section is shown:
| Sample # |
ppm A |
ppm B |
|
SKC MC
1
SKC MC 2
SKC MC 3
Supelco MC 1
Supelco MC 2
Supelco
MC 3 |
1.34
1.38
1.30
0.94
1.19
1.06 |
0.10
0.15
0.27
0.37
0.38
0.30 |
The block diagram of the major components of the
generation system is shown below. This system provided a means of
generating dynamic test atmospheres. The system consists of four essential
elements:
1) Flow, temperature, and humidity control system
2)
Phosphine vapor generating system
3) Mixing chamber
4)
Active sampling manifold
Figure 1
Figure
2
Storage Stability - Collected Samples
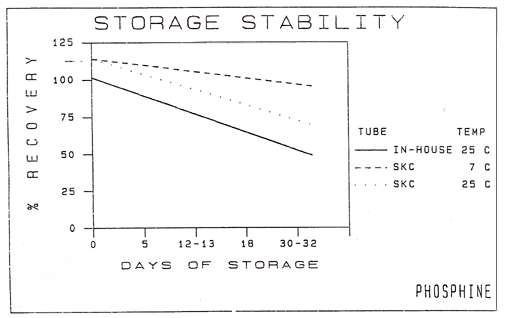
Figure 3
Effects of Humidity on Phosphine Sample Collection
Figure
4
Detection Limit Curve
Addendum 1
Comparison of
Different Lots of Carbon Bead |
The original evaluation of the treated carbon bead was
conducted with bead obtained from Union Carbide [(Tonawanda, NY) Note: The
carbon bead was called "Purasieve" by Union Carbide]. More recent lots
have been obtained from Kureha Chemical, MY. Recent lots of carbon bead
have been less efficient in collecting phosphine when using the sampling
and analytical parameters described in the method (11.1). A series of
experiments, were conducted to determine phosphine recoveries for
different lots and to find a lot that gave adequate
recoveries.
Procedure: A generation system
as mentioned in Section 2 was used to generate dynamic atmospheres of
phosphine. Sampling tubes were prepared at the laboratory or were obtained
commercially from SKC Inc. (Eighty Four, PA) or Supelco (Bellefonte, PA).
All samples were collected using the following conditions:
80% RH, 25°C, sample flow rate of
approximately 0.15 L/min Results: As shown below, the Union Carbide bead and the
Kureha Chemical Co. lot no. 820601 and 15161 carbon bead gave acceptable
recoveries for the collection of phosphine. The Supelco bead recovery was
slightly low at approximately 75%.
| Experiment
# |
|
Results |
|
|
|
| 1 |
|
OSHA-New
|
OSHA-Old
|
|
|
ppm,
PH3 |
0.74 |
2.68 |
|
|
|
Sampling
time = 60 min
Theoretical PH3 concentration = 2.70
ppm |
|
| 2a |
|
SKC-567
|
SKC-646
|
SKC-537
|
|
ppm,
PH3 |
0.23 |
0.26 |
0.66 |
| |
|
|
|
|
| 2b |
|
SKC-567
|
SKC-646
|
SKC-537
|
|
ppm,
PH3 |
0.17 |
0.18 |
0.41 |
|
|
Sampling time = 120
min
Theoretical PH3 concentration:
2a =
0.70 ppm
2b = 0.40 ppm |
|
Addendum (Continued)
Comparison of
Different Lots of Carbon Bead
| Experiment
# |
|
Results |
|
|
| 3 |
|
Supelco
|
OSHA-Old
|
|
ppm,
PH3 |
0.52 |
0.70 |
|
|
Sampling
time = 240 min
Theoretical PH3 concentration =
0.70 ppm |
|
| 4 |
|
JKC
|
SKC-537
|
|
ppm,
PH3 |
0.56* |
0.61 |
| Average value, n = 4 |
Sampling
time = 240 min
Theoretical PH3 concentration =
0.60 ppm |
|
| 5 |
|
JKC2
|
SKC-537
|
|
ppm,
PH3 |
1.25**
1.40*** |
1.41 |
** Sampling
rate 0.3 L/min
*** Sampling rate 0.15 L/min, n = 2 |
Sampling time = 120 min
Theoretical
PH3 concentration = 1.40 ppm |
|
| Identities |
Source |
|
OSHA-New:
OSHA-Old:
SKC-537:
SKC-567:
SKC-646:
Supelco:
JKC:
JKC2: |
Kureha Chemical Co. lot
no. G270R 77137
Union Carbide, lot no. unknown; used to
validate method ID-180
Same as OSHA-Old
Same as
OSHA-New
From SKC Inc., lot no. unknown
From Supelco, lot
no. 765-93, 20/40 mesh.
Kureha Chemical Co. (Japan) Grade
MU-AZ, lot no. 820601
Kureha Chemical Co. (Japan) Grade
MU-AZ, lot no. 15161 |
The "OSHA, Supelco,
and JKCII designated sampling tubes were impregnated with
potassium hydroxide and prepared in-house. The "SKC" sampling
tubes were prepared by SKC Inc. The "SKC 646" series is assumed
to be carbon bead recently obtained from Kureha and is similar
to OSHA-Nev. | Summary: The Kureha Chemical Co. lot
no. 820601 carbon bead appears acceptable to use for phosphine sampling
after it is impregnated with potassium hydroxide. The lot obtained from
Union Carbide is no longer available. Presumably, this lot had also
originated from Kureha Chemical.
Further testing using electron
microscopy and X-ray fluorescence to determine if any significant physical
differences existed among the lots of carbon bead have been inconclusive.
Subtle differences in physical structure and sulfur content were noted
between the lots. Greater collection efficiency was noted for those lots
showing the presence of small amounts of sulfur. Examination of the
physical character of the beads with a scanning electron microscope
revealed less surface fissures and cracks, and more smooth surface
indentations for the more efficient beads; however, at this point in time
it is unknown as to why lot differences exist for the collection retention
of phosphine. Personnel at Kureha Chemical Co. indicated the pitch used in
the bead production sometimes varies widely.
An additional study was
conducted to assess the ability of the Kureha Chemical Co. lot no. 820601
carbon bead to collect samples near the STEL concentration of 1 ppm
PH3 The bead was impregnated with KOH and prepared as mentioned
in the method. The generation system as described in Section 2 was used to
produce a dynamic atmosphere of approximately 1.3 ppm PH3 at
25°C and 80% RH. Samples were taken from the system first at different
flow rates to determine the potential for breakthrough. Two sampling tubes
were connected in series and samples were taken for 15-min at each of the
flow rates. Results are shovn below.
|
|
--ppm PH3
Found--- |
|
| Sample |
Flow Rate, L/min |
1st Tube |
2nd Tube |
% Breakthrough |
|
1
2
3
4 |
0.15
0.30
0.50
1.00 |
1.22
1.22
1.03
1.07 |
ND
ND
ND
0.07 |
0
0
0
6.1 | ND = None detectable < 0.012 ppm
PH3
The amount of breakthrough and sensitivity of the
analytical instrument for the amount of PH3 collected for
15-min was assessed. The sampling flow rate of 0.3 L/min and a sampling
time of 15-min was considered acceptable for STEL determinations of
PH3
Seven samples were then taken from the generation
system using the conditions mentioned above. A sampling flow rate of
approximately 0.3 L/min was used for 15-min.
| Sample |
PH3 ppm Found |
PH3 ppm Taken |
%
Recovery |
|
1
2
3
4
5
6
7 |
1.18
1.21
1.21
1.27
1.17
1.21
1.30 |
1.30
1.30
1.30
1.30
1.30
1.30
1.30 |
90.8
93.1
93.1
97.7
90.0
93.1
100.0 |
| |
|
|
|
n = 7
Mean = 1.22
Std Dev = 0.047
CV2 =
0.039 |
|
94.0 |
| |
|
|
|
| Overall Error (STEL) = 13.8% |
|
|
|

