ASBESTOS IN AIR
| Method No.: | ID-160 |
| Matrix: | Air |
| OSHA Permissible Exposure Limits Time Weighted Average: Excursion Level (30 minutes): |
0.1 fiber/cc 1.0 fiber/cc |
| Collection Procedure: | A known volume of air is drawn through a
|
| Recommended Sampling Rate: | 0.5 to 5.0 liters/minute (L/min) |
| Recommended Air
Volumes Minimum: Maximum: |
25 L 2,400 L |
| Analytical Procedure: | A portion of the sample filter is cleared and prepared for asbestos fiber counting by Phase Contrast Microscopy (PCM) at 400X. |
| Detection Limit: | 5.5 fibers/mm(2) or 0.001 fibers/cc (2,400 L Air Volume) |
| Precision CV: |
0.12 (at 100 fibers/mm(2)) |
| Method Classification: | Validated Method |
| Date (Date Revised): | July 1988 (July 1997) |
| Physical Scientist: | Daniel T. Crane |
Commercial manufacturers and products mentioned in this method are for
descriptive use only and do not constitute endorsements by
OSHA Technical Center
Salt Lake City, Utah
1. Introduction
This method describes the collection of airborne asbestos fibers using
calibrated sampling pumps with
Asbestos: A term for naturally occurring fibrous minerals. Asbestos includes chrysotile, crocidolite, amosite (cummingtonite-grunerite asbestos), tremolite asbestos, actinolite asbestos, anthophyllite asbestos, and any of these minerals that have been chemically treated and/or altered. The precise chemical formulation of each species will vary with the location from which it was mined. Nominal compositions are listed:
| Chrysotile | Mg(3)Si(2)O(5)(OH)(4) |
| Crocidolite | Na(2)Fe(3)(2+)Fe(2)(3+)Si(8)O(22)(OH)(2) |
| Amosite | (Mg,Fe)(7)Si(8)O(22)(OH)(2) |
| Tremolite-actinolite series | Ca(2)(Mg,Fe)(5)Si(8)O(22)(OH)(2) |
| Anthophyllite | (Mg,Fe)(7)Si(8)O(22)(OH)(2) |
Asbestos Fiber: A fiber of asbestos which meets the criteria
specified below for a fiber.
Aspect Ratio: The ratio of the
length of a fiber to it's diameter (e.g. 3:1, 5:1 aspect ratios).
Cleavage Fragments: Mineral particles formed by comminution of
minerals, especially those characterized by parallel sides and a moderate
aspect ratio (usually less than 20:1).
Detection Limit: The
number of fibers necessary to be 95% certain that the result is greater
than zero.
Differential Counting: The term applied to the
practice of excluding certain kinds of fibers from the fiber count because
they do not appear to be asbestos.
Fiber: A particle that is 5
µm or longer, with a
Field: The area within the graticule circle that is
superimposed on the microscope image.
Set: The samples which
are taken, submitted to the laboratory, analyzed, and for which, interim
or final result reports are generated.
Tremolite, Anthophyllite,
and Actinolite: The
Walton-Beckett
Graticule: An eyepiece graticule specifically designed for asbestos
fiber counting. It consists of a circle with a projected diameter of 100 ±
2 µm (area of about 0.00785 mm(2)) with a crosshair having
- 1.1. History
Early surveys to determine asbestos exposures were conducted using impinger counts of total dust with the counts expressed as million particles per cubic foot (8.1.). The British Asbestos Research Council (8.2.) recommended filter membrane counting in 1969. In July 1969, the Bureau of Occupational Safety and Health published a filter membrane method for counting asbestos fibers in the United States (8.3.). This method was refined by NIOSH and published as P & CAM 239 (8.4.). On May 29, 1971, OSHA specified filter membrane sampling with phase contrast counting for evaluation of asbestos exposures at work sites in the United States (8.5.). The use of this technique was again required by OSHA in 1986 (8.6.). Phase contrast microscopy has continued to be the method of choice for the measurement of occupational exposure to asbestos (8.7.).
1.2. Principle
Air is drawn through a MCE filter to capture airborne asbestos fibers. A wedge shaped portion of the filter is removed, placed on a glass microscope slide and made transparent. A measured area (field) is viewed by PCM. All the fibers meeting defined criteria for asbestos are counted and considered a measure of the airborne asbestos concentration.
1.3. Advantages and Disadvantages
There are four main advantages of PCM over other methods:
1) The technique is specific for fibers. Phase contrast is a fiber
counting technique which excludes
2) The technique is inexpensive and does not require specialized knowledge to carry out the analysis for total fiber counts.
3) The analysis is quick and can be performed
4) The technique has continuity with historical epidemiological
studies so that estimates of expected disease can be inferred from
The main disadvantage of PCM is that it does not positively identify asbestos fibers. Other fibers which are not asbestos may be included in the count unless differential counting is performed. This requires a great deal of experience to adequately differentiate asbestos from non-asbestos fibers. Positive identification of asbestos must be performed by polarized light or electron microscopy techniques. A further disadvantage of PCM is that the smallest visible fibers are about 0.2 µm in diameter while the finest asbestos fibers may be as small as 0.02 µm in diameter. For some exposures, substantially more fibers may be present than are actually counted.
1.4. Workplace Exposure
Asbestos is used by the construction industry in such products as
shingles, floor tiles, asbestos cement, roofing felts, insulation and
acoustical products.
About 95% of the asbestos in commercial use in the United States is chrysotile. Crocidolite and amosite make up most of the remainder. Anthophyllite and tremolite or actinolite are likely to be encountered as contaminants in various industrial products.
1.5. Physical Properties
Asbestos fiber possesses a high tensile strength along its axis, is chemically inert, non-combustible, and heat resistant. It has a high electrical resistance and good sound absorbing properties. It can be weaved into cables, fabrics or other textiles, and also matted into asbestos papers, felts, or mats.
1.6. Toxic Effects
Information contained in this section is a synopsis of current knowledge of the physiological effects of asbestos and is not intended as a basis for OSHA policy.
- Some possible physiologic results of respiratory exposure to
asbestos are mesothelioma of the pleura or peritoneum, interstitial
fibrosis, asbestosis, pneumoconiosis, or respiratory cancer (8.8.).
The possible consequences of asbestos exposure are further detailed in
reference 8.8 or in the asbestos standard preamble (8.6.).
2. Range and Detection Limit
- 2.1. The ideal counting range on the filter is 100 to 1,300
fibers/mm(2). With a Walton-Beckett graticule this range is
equivalent to 0.8 to 10 fibers/field. Using NIOSH counting statistics
(8.9.), a count of 0.8 fibers/field would give an approximate
coefficient of variation (CV) of 0.13.
2.2. The detection limit for this method is 4.0 fibers per 100 fields or 5.5 fibers/mm(2). This was determined using an equation to estimate the maximum CV possible at a specific concentration (95% confidence) and a Lower Control Limit of zero. The CV value was then used to determine a corresponding concentration from historical CV vs fiber relationships. As an example:
Lower Control Limit (95% Confidence) = AC - 1.645(CV)(AC)
Where:
AC = Estimate of the airborne fiber concentration
(fibers/cc)
Setting the Lower Control Limit = 0 and solving for
CV:
0 = AC - 1.645(CV)(AC)
CV = 0.61
This value was compared with CV vs. count curves. The count at
which CV = 0.61 for Leidel-Busch counting statistics (8.9.) or for an
OSHA Salt Lake Technical Center (OSHA-SLTC) CV curve (see Appendix A
for further information) was 4.4 fibers or 3.9 fibers per 100 fields,
respectively. Although a lower detection limit of 4 fibers per 100
fields is supported by the
3. Method Performance - Precision and Accuracy
Precision is dependent upon the total number of fibers counted and the uniformity of the fiber distribution on the filter. A general rule is to count at least 20 and not more than 100 fields. The count is discontinued when 100 fibers are counted, provided that 20 fields have already been counted. Counting more than 100 fibers results in only a small gain in precision. As the total count drops below 10 fibers, an accelerated loss of precision is noted (8.9.).
At this time, there is no known method to determine the absolute
accuracy of the asbestos analysis. Results of samples prepared through the
Proficiency Analytical Testing (PAT) Program and analyzed by the
4. Interferences
Fibrous substances, if present, may interfere with asbestos analysis.
Some common fibers are:
| fiber glass | perlite veins |
| anhydrite | plant fibers |
| gypsum | some synthetic fibers |
| membrane structures | sponge spicules and diatoms |
| microorganisms | wollastonite |
The use of electron microscopy or optical tests such as polarized light, and dispersion staining may be used to differentiate these materials from asbestos when necessary.
5. Sampling
- 5.1. Equipment
- 5.1.1. Sample assembly (The assembly is shown in Figure 3):
Conductive filter holder consisting of a
| NOTES: | a) DO NOT b) Fully conductive cassettes are required to reduce fiber loss to the sides of the cassette due to electrostatic attraction. c) Purchase filters which have been selected by the manufacturer for asbestos counting or analyze representative filters for fiber background before use. Discard the filter lot if more than 4 fibers/100 fields are found. d) To decrease the possibility of contamination, the sampling system (filter-backup pad-cassette) for asbestos is usually preassembled by the manufacturer. e) Other cassettes such as the |
- 5.1.2. Gel bands for sealing cassettes.
5.1.3 Sampling pump: Each pump must be a battery operated,
5.1.4. Flexible tubing,
5.1.5. Pump calibration: Stopwatch and bubble tube/burette or electronic meter.
5.2. Sampling Procedure
- 5.2.1. Seal the point where the base and cowl of each cassette
meet (see Figure 3) with a gel band or tape.
5.2.2. Charge the pumps completely before beginning.
5.2.3. Connect each pump to a calibration cassette with an
appropriate length of
5.2.4. Select an appropriate flow rate for the situation being monitored. The sampling flow rate must be between 0.5 and 5.0 L/min for personal sampling and is commonly set between 1 and 2 L/min. Always choose a flow rate that will not produce overloaded filters.
5.2.5. Calibrate each sampling pump before and after sampling with a calibration cassette in-line (Note: This calibration cassette should be from the same lot of cassettes used for sampling). Use a primary standard (e.g. bubble burette) to calibrate each pump. If possible, calibrate at the sampling site.
| NOTE: | If sampling site calibration is not possible, environmental influences may affect the flow rate. The extent is dependent on the type of pump used. Consult with the pump manufacturer to determine dependence on environmental influences. If the pump is affected by temperature and pressure changes, use the formula in Appendix B to calculate the actual flow rate. |
- 5.2.6. Connect each pump to the base of each sampling cassette
with flexible tubing. Remove the end cap of each cassette and take
each air sample open face (see Figure 3). Assure that each
sample cassette is held open side down in the employee's breathing
zone during sampling. The distance from the nose/mouth of the
employee to the cassette should be about 10 cm. Secure the cassette
on the collar or lapel of the employee using spring clips or other
similar devices.
5.2.7. A suggested minimum air volume when sampling to determine TWA compliance is 25 L. For Excursion Limit (30 min sampling time) evaluations, a minimum air volume of 48 L is recommended.
5.2.8. The most significant problem when sampling for asbestos is overloading the filter with non-asbestos dust. Suggested maximum air sample volumes for specific environments are:
|
|
|
| Asbestos removal operations (visible dust) | 100 |
| Asbestos removal operations (little dust) | 240 |
| Office environments | 400 to 2,400 |
Do not overload the filter with dust. High levels of
- While sampling, observe the filter with a small flashlight. If
there is a visible layer of dust on the filter, stop sampling,
remove and seal the cassette, and replace with a new sampling
assembly. The total dust loading should not exceed 1 mg.
5.2.9. Blank samples are used to determine if any contamination has occurred during sample handling. Prepare two blanks for the first 1 to 20 samples. For sets containing greater than 20 samples, prepare blanks as 10% of the samples. Handle blank samples in the same manner as air samples with one exception: Do not draw any air through the blank samples. Open the blank cassette in the place where the sample cassettes are mounted on the employee. Hold it open for about 30 seconds. Close and seal the cassette appropriately. Store blanks for shipment with the sample cassettes.
5.2.10. Immediately after sampling, close and seal each cassette with the base and plastic plugs. Do not touch or puncture the filter membrane as this will invalidate the analysis.
5.2.11. Attach a seal (OSHA-21 or equivalent) around each
cassette in such a way as to secure the end cap plug and base plug.
Tape the ends of the seal together since the seal is not long enough
to be wrapped
5.3. Sample Shipment
5.3.1. Send the samples to the laboratory with paperwork requesting asbestos analysis. List any known fibrous interferences present during sampling on the paperwork. Also, note the workplace operation(s) sampled.
5.3.2. Secure and handle the samples so that they will not rattle during shipment nor be exposed to static electricity. Do not ship samples in expanded polystyrene peanuts, vermiculite, paper shreds, or excelsior. Tape sample cassettes to sheet bubbles and place in a container that will cushion the samples without rattling.
5.3.3. To avoid the possibility of sample contamination, always ship bulk samples in separate mailing containers.
6. Analysis
- 6.1. Safety Precautions
- 6.1.1. Acetone is extremely flammable and precautions must be
taken not to ignite it. Avoid using large containers or quantities
of acetone. Transfer the solvent in a ventilated laboratory hood. Do
not use acetone near any open flame. For generation of acetone
vapor, use a spark free heat source.
6.1.2. Any asbestos spills should be cleaned up immediately to
prevent dispersal of fibers. Prudence should be exercised to avoid
contamination of laboratory facilities or exposure of personnel to
asbestos. Asbestos spills should be cleaned up with wet methods
and/or a High Efficiency
6.2. Equipment
- 6.2.1. Phase contrast microscope with binocular or trinocular
head.
6.2.2. Widefield or Huygenian 10X eyepieces (NOTE: The eyepiece containing the graticule must be a focusing eyepiece. Use a 40X phase objective with a numerical aperture of 0.65 to 0.75).
6.2.3. Kohler illumination (if possible) with green or blue filter.
6.2.4.
6.2.5. Mechanical stage. A rotating mechanical stage is convenient for use with polarized light.
6.2.6. Phase telescope.
6.2.7. Stage micrometer with 0.01-mm subdivisions.
6.2.8.
6.2.9. Precleaned glass slides, 25 mm X 75 mm. One end can be
frosted for convenience in writing sample numbers, etc., or
6.2.10. Cover glass #1½.
6.2.11. Scalpel (#10, curved blade).
6.2.12. Fine tipped forceps.
6.2.13. Aluminum block for clearing filter (see Appendix D and Figure 4).
6.2.14. Automatic adjustable pipette,
6.2.15. Micropipette, 5 µL.
6.3. Reagents
- 6.3.1. Acetone (HPLC grade).
6.3.2. Triacetin (glycerol triacetate).
6.3.3. Lacquer or nail polish.
6.4. Standard Preparation
A way to prepare standard asbestos samples of known concentration has not been developed. It is possible to prepare replicate samples of nearly equal concentration. This has been performed through the PAT program. These asbestos samples are distributed by the AIHA to participating laboratories.
Since only about
6.5. Sample Mounting
- The objective is to produce samples with a smooth (non-grainy)
background in a medium with a refractive index of approximately 1.46.
The technique below collapses the filter for easier focusing and
produces permanent mounts which are useful for quality control and
interlaboratory comparison.
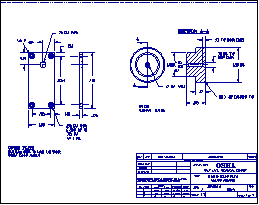
An aluminum block or similar device is required for sample preparation. A drawing is shown in Figure 4.
- 6.5.1. Heat the aluminum block to about 70°C. The hot block
should not be used on any surface that can be damaged by either the
heat or from exposure to acetone.
6.5.2. Ensure that the glass slides and cover glasses are free of dust and fibers.
6.5.3. Remove the top plug to prevent a vacuum when the cassette is opened. Clean the outside of the cassette if necessary. Cut the seal and/or tape on the cassette with a razor blade. Very carefully separate the base from the extension cowl, leaving the filter and backup pad in the base.
6.5.4. With a rocking motion cut a triangular wedge from the
filter using the scalpel. This wedge should be
6.5.5. Place the tip of the micropipette containing about 200 µL acetone into the aluminum block. Insert the glass slide into the receiving slot in the aluminum block. Inject the acetone into the block with slow, steady pressure on the plunger while holding the pipette firmly in place. Wait 3 to 5 seconds for the filter to clear, then remove the pipette and slide from the aluminum block.
6.5.6. Immediately (less than 30 seconds) place 2.5 to 3.5 µL of triacetin on the filter (NOTE: Waiting longer than 30 seconds will result in increased index of refraction and decreased contrast between the fibers and the preparation. This may also lead to separation of the cover slip from the slide).
6.5.7. Lower a cover slip gently onto the filter at a slight angle to reduce the possibility of forming air bubbles. If more than 30 seconds have elapsed between acetone exposure and triacetin application, glue the edges of the cover slip to the slide with lacquer or nail polish.
6.5.8. If clearing is slow, warm the slide for 15 min on a hot plate having a surface temperature of about 50°C to hasten clearing. The top of the hot block can be used if the slide is not heated too long.
6.5.9. Counting may proceed immediately after clearing and mounting are completed.
6.6. Sample Analysis
Completely align the microscope according to the manufacturer's instructions. Then, align the microscope using the following general alignment routine at the beginning of every counting session and more often if necessary.
6.6.1. Alignment
1) Clean all optical surfaces. Even a small amount of dirt can significantly degrade the image.
2) Rough focus the objective on a sample.
3) Close down the field iris so that it is visible in the field of view. Focus the image of the iris with the condenser focus. Center the image of the iris in the field of view.
4) Install the phase telescope and focus on the phase rings. Critically center the rings. Misalignment of the rings results in astigmatism which will degrade the image.
5) Place the
6.6.2. Counting Fibers
1) Place the prepared sample slide on the mechanical stage of the microscope. Position the center of the wedge under the objective lens and focus upon the sample.
2) Start counting from one end of the wedge and progress along a radial line to the other end (count in either direction from perimeter to wedge tip). Select fields randomly, without looking into the eyepieces, by slightly advancing the slide in one direction with the mechanical stage control.
3) Continually scan over a range of focal planes (generally the upper 10 to 15 µm of the filter surface) with the fine focus control during each field count. Spend at least 5 to 15 seconds per field.
4) Most samples will contain asbestos fibers with fiber diameters less than 1 µm. Look carefully for faint fiber images. The small diameter fibers will be very hard to see. However, they are an important contribution to the total count.
5) Count only fibers equal to or longer than 5 µm. Measure the length of curved fibers along the curve.
6) Count fibers which have a length to width ratio of 3:1 or greater.
7) Count all the fibers in at least 20 fields. Continue counting until either 100 fibers are counted or 100 fields have been viewed; whichever occurs first. Count all the fibers in the final field.
8) Fibers lying entirely within the boundary of the
9) Count bundles of fibers as one fiber unless individual fibers can be clearly identified and each individual fiber is clearly not connected to another counted fiber. See Figure 2 for counting conventions.
10) Record the number of fibers in each field in a consistent way such that filter non-uniformity can be assessed.
11) Regularly check phase ring alignment.
12) When an agglomerate (mass of material) covers more than 25% of the field of view, reject the field and select another. Do not include it in the number of fields counted.
13) Perform a "blind recount" of 1 in every 10 filter wedges
(slides).
6.7. Fiber Identification
As previously mentioned in Section 1.3., PCM does not provide positive confirmation of asbestos fibers. Alternate differential counting techniques should be used if discrimination is desirable. Differential counting may include primary discrimination based on morphology, polarized light analysis of fibers, or modification of PCM data by Scanning Electron or Transmission Electron Microscopy.
A great deal of experience is required to routinely and correctly perform differential counting. It is discouraged unless it is legally necessary. Then, only if a fiber is obviously not asbestos should it be excluded from the count. Further discussion of this technique can be found in reference 8.10.
"WHEN IN DOUBT, COUNT."
- 6.8. Analytical Recommendations - Quality Control System
6.8.1. All individuals performing asbestos analysis must have taken the NIOSH course for sampling and evaluating airborne asbestos or an equivalent course.
6.8.2. Each laboratory engaged in asbestos counting shall set up a slide trading arrangement with at least two other laboratories in order to compare performance and eliminate inbreeding of error. The slide exchange occurs at least semiannually. The round robin results shall be posted where all analysts can view individual analyst's results.
6.8.3. Each laboratory engaged in asbestos counting shall participate in the Proficiency Analytical Testing Program, the Asbestos Analyst Registry or equivalent.
6.8.4. Each analyst shall select and count prepared slides from a "slide bank". These are quality assurance counts. The slide bank shall be prepared using uniformly distributed samples taken from the workload. Fiber densities should cover the entire range routinely analyzed by the laboratory. These slides are counted blind by all counters to establish an original standard deviation. This historical distribution is compared with the quality assurance counts. A counter must have 95% of all quality control samples counted within three standard deviations of the historical mean. This count is then integrated into a new historical mean and standard deviation for the slide.
The analyses done by the counters to establish the slide bank may be used for an interim quality control program if the data are treated in a proper statistical fashion.
7. Calculations
- 7.1. Calculate the estimated airborne asbestos fiber concentration
on the filter sample using the following formula:
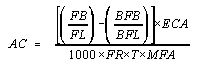
where:
AC = Airborne fiber concentration
FB = Total number of fibers greater than 5 µm counted
FL = Total number of fields counted on the filter
BFB = Total number of fibers greater than 5 µm counted in the blank
BFL = Total number of fields counted on the blank
ECA = Effective collecting area of filter (385 mm(2) nominal for a
FR = Pump flow rate (L/min)
MFA = Microscope count field area (mm(2)). This is 0.00785 mm(2) for a
T = Sample collection time (min)
1,000 = Conversion of L to cc
| NOTE: | The collection area of a filter is seldom equal to 385 mm(2). It is appropriate for laboratories to routinely monitor the exact diameter using an inside micrometer. The collection area is calculated according to the formula: |
- 7.2.
Since a given analyst always has the same interpupillary distance, the number of fields per filter for a particular analyst will remain constant for a given size filter. The field size for that analyst is constant (i.e. the analyst is using an assigned microscope and is not changing the reticle).
For example, if the exposed area of the filter is always 385 mm(2) and the size of the field is always 0.00785 mm(2), the number of fields per filter will always be 49,000. In addition it is necessary to convert liters of air to cc. These three constants can then be combined such that ECA/(1,000 X MFA) = 49. The previous equation simplifies to:
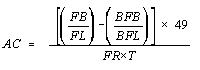
7.3. Recount Calculations
As mentioned in step 13 of Section 6.6.2., a "blind recount" of 10% of the slides is performed. In all cases, differences will be observed between the first and second counts of the same filter wedge. Most of these differences will be due to chance alone, that is, due to the random variability (precision) of the count method. Statistical recount criteria enables one to decide whether observed differences can be explained due to chance alone or are probably due to systematic differences between analysts, microscopes, or other biasing factors.
The following recount criterion is for a pair of counts that
estimate AC in fibers/cc. The criterion is given at the
Reject a pair of counts if:
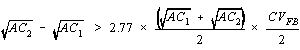
- Where:
AC(1) = lower estimated airborne fiber concentration
AC(2) = higher estimated airborne fiber concentration
CV(FB) = Pooled average CV for the two concentration estimates:
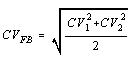
CV(1) = Coefficient of variation associated with
the lower count
CV(2) = Coefficient of variation
associated with the higher count
Coefficients of variation (CV) may be determined as in Appendix A of
this method (ID-160) or as discussed in NIOSH Method 7400.
- If a pair of counts are rejected by this criterion then, recount
the rest of the filters in the submitted set. Apply the test and
reject any other pairs failing the test. Rejection shall include a
memo to the industrial hygienist stating that the sample failed a
statistical test for homogeneity and the true air concentration may be
significantly different than the reported value.
7.4. Reporting Results
Report results to the industrial hygienist as fibers/cc. Use two significant figures. If multiple analyses are performed on a sample, an average of the results is to be reported unless any of the results can be rejected for cause.
8. References
- 8.1. Dreesen, W.C., et al, U.S. Public Health Service: A
Study of Asbestosis in the Asbestos Textile Industry, (Public
Health Bulletin No. 241), US Treasury Dept., Washington, DC, 1938.
8.2. Asbestos Research Council: The Measurement of Airborne Asbestos Dust by the Membrane Filter Method (Technical Note), Asbestos Research Council, Rockdale, Lancashire, Great Britain, 1969.
8.3. Bayer, S.G., Zumwalde, R.D., Brown, T.A., Equipment and Procedure for Mounting Millipore Filters and Counting Asbestos Fibers by Phase Contrast Microscopy, Bureau of Occupational Health, U.S. Dept. of Health, Education and Welfare, Cincinnati,OH,1969.
8.4. NIOSH Manual of Analytical Methods, 2nd ed., Vol. 1
(DHEW/NIOSH Pub. No.
8.5. Asbestos, Code of Federal Regulations 29 CFR 1910.1001. 1971.
8.6. Occupational Exposure to Asbestos, Tremolite,
Anthophyllite, and Actinolite. Final Rule, Federal Register 51:
119 (20 June 1986).
8.7. Asbestos, Tremolite, Anthophyllite, and Actinolite,
Code of Federal Regulations 1910.1001. 1988.
8.8. Criteria for a Recommended Standard -- Occupational
Exposure to Asbestos (DHEW/NIOSH Pub. No. HSM
8.9. Leidel, N.A., Bayer,S.G., Zumwalde, R.D.,Busch,
K.A., USPHS/NIOSH Membrane Filter Method for Evaluating
Airborne Asbestos Fibers (DHEW/NIOSH Pub. No.
8.10. Dixon, W.C., Applications of Optical Microscopy in
Analysis of Asbestos and Quartz, Analytical Techniques in
Occupational Health Chemistry, edited by D.D. Dollberg and A.W.
Verstuyft. Wash. D.C.: American Chemical Society, (ACS Symposium
Series 120) 1980. pp.
8.11. Abell, M. T., et al.,The Quality of Fiber Count
Data, Appl. Ind. Hyg. Vol 4 No.11, November 1989, pp.
Appendix A
The OSHA asbestos regulations require each laboratory to establish a
quality control program. The following is presented as an example of how
the
Data for the CV curve shown below is from 395 samples collected during OSHA compliance inspections and analyzed from October 1980 through April 1986.
Each sample was counted by 2 to 5 different counters independently of one another. The standard deviation and the CV statistic was calculated for each sample. This data was then plotted on a graph of CV vs. fibers/mm(2). A least squares regression was performed using the following equation:
- CV = antilog(10)[A(log(10)(x))(2)
+ B(log(10)(x)) + C]
| where: | x = the number of fibers/mm(2) |
Application of least squares gave:
- A = 0.182205
B = -0.973343
C = 0.327499
Using these values, the equation becomes:
CV = antilog(10)[0.182205(log(10)(x))(2) - 0.973343(log(10)(x)) + 0.327499]

Figure 1: CV curve generated from OSHA Salt Lake
Technical Center data.
Appendix B
Sampling Pump Flow Rate Corrections
This correction is used if a difference greater than 5% in ambient
temperature and/or pressure is noted between calibration and sampling
sites and the pump does not compensate for the differences.

Where:
- Q(act) = actual flow rate
Q(cal) = calibrated flow rate (if a rotameter was used, the rotameter value)
P(cal) = uncorrected air pressure at calibration
P(act) = uncorrected air pressure at sampling site
T(act) = temperature at sampling site (K)
T(cal) = temperature at calibration (K)
Walton-Beckett Graticule
When ordering the Graticule for asbestos counting, specify the exact disc diameter needed to fit the ocular of the microscope and the diameter (mm) of the circular counting area. Instructions for measuring the dimensions necessary are listed:
- Insert any available graticule into the focusing eyepiece and focus so that the graticule lines are sharp and clear.
- Align the microscope.
- Place a stage micrometer on the microscope object stage and focus the microscope on the graduated lines.
- Measure the magnified grid length, PL (µm), using the stage micrometer.
- Remove the graticule from the microscope and measure its actual grid length, AL (mm). This can be accomplished by using a mechanical stage fitted with verniers, or a jeweler's loupe with a direct reading scale.
- Let D = 100 µm. Calculate the circle diameter, d(c) (mm),
for the
Walton-Beckett graticule and specify the diameter when making a purchase:dc = AL × D
PLExample: If PL = 108 µm, AL = 2.93 mm and D = 100 µm, then, dc = 2.93 × 100
108= 2.71 mm - Each
eyepiece-objective-reticle combination on the microscope must be calibrated. Should any of the three be changed (by zoom adjustment, disassembly, replacement, etc.), the combination must be recalibrated. Calibration may change if interpupillary distance is changed.Measure the field diameter, D (acceptable range: 100 ± 2 µm) with a stage micrometer upon receipt of the graticule from the manufacturer. Determine the field area (mm(2)).
- Field Area = p(D/2)(2)
If D = 100 µm = 0.1 mm, then
Field Area = p(0.1 mm/2)(2) = 0.00785 mm(2)
The Graticule is available from: Graticules Ltd., Morley Road,
Tonbridge TN9 IRN, Kent, England (Telephone
Appendix D
Aluminum Block
Diagrams of the block are provided in Figure 4.
The cartridge thermostat and heater used for this block have the following dimensions:
| Diameter: | 1/2" |
| Cartridge length: | 2 3/8" |
These heating units were obtained from:
- Vulcan Electric Company
Kezar Falls, Maine 04047
(207)-625-3231
| Thermostat part number: | N1A1C2 |
| Heater part number: | C516 |
MENTION OF ANY PRODUCT NAME IN THIS DOCUMENT IS FOR INFORMATION ONLY AND DOES NOT CONSTITUTE ENDORSEMENT BY DOL-OSHA.

Figure 3: Walton-Beckett Graticule with some explanatory fibers.
Counts for the fibers in the figure
| Structure number | Count | Explanation |
|
| ||
| 1 to 6 | 1 | single fibers all contained within the circle |
| 7 | ½ | fiber crosses circle once |
| 8 | 0 | fiber too short |
| 9 | 2 | two crossing fibers |
| 10 | 0 | fiber outside graticule |
| 11 | 0 | fiber crosses graticule twice |
| 12 | ½ | although split, fiber only crosses once |

Figure 3. Exploded view of an asbestos sampling
casette.
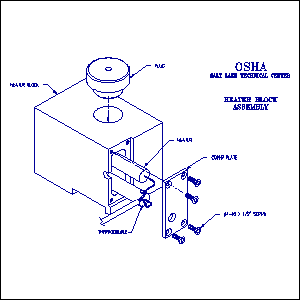 Figure 4: Engineering Drawings for the aluminum "Hot
Block" as used at the Salt Lake Technical Center.
Figure 4: Engineering Drawings for the aluminum "Hot
Block" as used at the Salt Lake Technical Center.