HEXAVALENT CHROMIUM
| Method Number: | ID-103 |
| Matrix: | Air |
| OSHA Permissible Exposure Limits Chromic acid and chromates as CrO3 (Final Rule Limit): |
0.1 mg/m3 (Ceiling) |
| Chromic acid and chromates as CrO3 (Transitional Limit): |
0.1 mg/m3 (Time Weighted Average) |
| Collection Device: | An air sample is collected on a 37-mm diameter polyvinyl
chloride filter |
| Recommended Sampling Rate: | 2 L/min |
| Recommended Air Volume Range: | 30 to 960 L |
| Analytical Procedure: | The chromium (VI) is extracted from the filter using a carbonate/bicarbonate buffer solution and then analyzed by differential pulse polarography. |
| Detection Limits Qualitative: |
0.006 mg/m3 as CrO3 (30-L air sample) |
| Quantitative: | 0.019 mg/m3 as CrO3 (30-L air sample) |
| Precision and Accuracy Validation Range: |
0.1 to 0.6 mg/m3 as CrO3 (30-L sample) |
| CV1 Range: | 0.012 to 0.019 |
| Bias Range: | +0.012 to +0.053 |
| Overall Error Range: | ¦2.7 to ¦8.7% |
| Method Classification: | Validated Method |
| Chemist: | James Ku |
| Date (Date Revised): | 1982 (February, 1990) |
Commercial manufacturers and products mentioned in this method are for descriptive use only and do not constitute endorsements by USDOL-OSHA.
Similar products from other sources can be substituted.
OSHA Technical Center
Salt Lake City, Utah
1. Introduction
This method describes the air sampling and subsequent analysis of workplace exposures to chromic acid and chromate compounds. Analysis is conducted by differential pulse polarography (DPP).
- 1.1. History
This method has been developed at the OSHA Salt Lake City Technical
Center
The classical method of Cr(VI) analysis is colorimetry using
The extraction of Cr(VI) in basic solution and subsequent analysis by colorimetry using DPC has been reported (8.3.). The extraction technique used in this method is a modification of that suggested in reference 8.3.
In this method the analytical technique for Cr(VI) is DPP. Polarographic techniques have been previously reported for the analysis of chromium species (8.5., 8.6.).
1.2. Principle
- 1.2.1. An air sample is collected on a 37-mm polyvinyl chloride
(PVC) filter [Note: Cellulose ester filters are unacceptable because
they may react with and reduce the hexavalent chromium [Cr(VI)]
species
1.2.2. The reaction between chromates and carbonate is illustrated by the following equation (8.3.):
MCrO4 + CO3(2 »)< ----> MCO3 + CrO4(2 »)
where M = metals (i.e. lead, zinc, cadmium, ...)
In the presence of a large excess of carbonate, equilibrium is quantitatively shifted to the right. The chromate compounds (soluble and insoluble) are converted to their corresponding carbonates.
1.3. Advantages and Disadvantages
- 1.3.1. The analysis is specific for Cr(VI) in the presence of
Cr(III). Other reducing substances, such as magnetite
(Fe3O4) do not
appear to significantly interfere (8.8.).
1.3.2. In addition to the Cr(VI) analysis, it is possible to determine other soluble compounds such as lead and zinc salts in the same solution.
1.3.3. By using alkaline extraction conditions (pH 10 to 11), sample recovery is improved by preventing Cr(VI) losses which may occur in a more acidic extraction media. Both water soluble and insoluble Cr(VI) compounds are soluble in this alkaline extraction medium.
1.3.4. The sensitivity is adequate for measuring workplace atmospheric concentrations of Cr(VI) and is less sensitive to interferences noted when using the colorimetric/DPC procedure (8.2., 8.7.). Potential interferences with the polarographic determination may be rendered insignificant by altering analytical conditions such as changing the supporting electrolyte solution.
1.3.5. Polarographic instruments have a wide analytical range. This diminishes the need for withdrawing aliquots or diluting the samples in order to be within the linear analytical range of the instrument.
1.3.6. A disadvantage is the polarographic instrument may not be available in some analytical laboratories; however, the extracted samples may be acidified and then analyzed using a modified colorimetric/DPC method (please see Section 7 of reference 8.8. for further information). Spiked samples using compounds known to be present in the sample matrix should be taken through this alternate procedure first to determine if any loss of Cr(VI) occurs during acidification. Detection limits should also be determined.
1.4. Uses
Occupations having a potential exposure to compounds containing Cr(VI) as well as a list of different chromate compounds are listed in reference 8.10.
2. Range and Detection Limit (8.8.)
- 2.1. This method was validated using insoluble and soluble
chromate compounds. The compounds used were lead, zinc, calcium, and
potassium chromates. Filter samples were spiked with about 2 to 9 µg
[as Cr(VI)], prepared, and then diluted to
| 30-L air sample | 0.1 to 0.6 mg/m3 as CrO3 | |
| 840-L air sample | 0.005 to 0.02 mg/m3 as CrO3 |
This method has the sensitivity necessary to determine compliance
with either the OSHA Transitional or the Final Rule PEL. Samples for
Final Rule determinations should be taken with at least
2.2. The qualitative and quantitative detection limits for 10-mL sample solution volumes were 0.19 µg and 0.58 µg as CrO3, respectively.
3. Method Performance (8.8.)
The DPP analytical method has been evaluated using a time weighted
average concentration of 0.009 mg/m3 as
CrO3
- 3.1. The pooled analytical coefficients of variation
(CV1) and recoveries at 0.5, 1, and 2 times
this concentration for specific chromate compounds were:
| Compound |
CV1 |
Recovery | ||
| Lead chromate (PbCrO4) | 0.012 | 100.3% | ||
| Zinc chromate (4ZnO+ CrO3.3H2O)* | 0.017 | 105.3% | ||
| Calcium chromate (CaCrO4) | 0.015 | 101.9% | ||
| Potassium chromate (K2CrO4) | 0.019 | 101.2% | ||
| * Molecular formula was confirmed by X-ray diffraction (8.8.) | ||||
3.2. A comparison of methods using spiked samples containing PbCrO4 showed that results obtained by a modified colorimetric/DPC method were duplicated for the DPP method. There was no significant bias between the two methods (8.8.).
3.3. A collection efficiency of 0.945 ¦ 0.035 has been previously determined for chromic acid mist collected on PVC filters (8.11.).
3.4. Quality control samples were prepared by spiking aqueous
solutions of potassium dichromate on PVC filters. These samples were
analyzed along with survey samples at
| Samples (N) | 282 | |
| Average recovery | 94.1% | |
| CV1 | 0.10 |
4. Interferences
- 4.1. Reducing species such as Cr(III) or magnetite
(Fe3O4) in an
excess of 10:1 or 50:1, respectively, over Cr(VI) did not produce a
significant interference with this method (8.8.).
4.2. The effect of many interferences can be minimized by changing
the operating conditions of the polarograph. Additional polarographic
confirmation of a cation in a sample may be performed in a second
electrolyte and observing if the new
5. Sampling
- 5.1. Sampling Equipment (Note: Bulk samples can be collected and
analyzed. Filter or wipe samples collected on cellulose or
cellulose esters are unacceptable due to chromate species
instability on these media.)
- 5.1.1. Sample assembly:
Filter holder consisting of a two- or three-piece cassette,
Backup pad, 37-mm,
cellulose.
Membrane filter, PVC, 37-mm, 5-µm pore size [part no.
625413, Mine Safety Appliances (MSA), Pittsburgh, PA or cat. no.
5.1.2. Gel bands (Omega Specialty Instrument Co., Chelmsford, MA) for sealing cassettes.
5.1.3. Sampling pumps capable of sampling at 2 L/min.
5.1.4. Assorted flexible tubing.
5.1.5. Stopwatch and bubble tube or meter for pump calibration.
5.1.6. Scintillation vials (for bulk samples), 20-mL, part no. 74515 or 58515, (Kimble, Div. of Owens-Illinois Inc., Toledo, OH) with polypropylene or Teflon cap liners.
5.2. Sampling Procedure
- 5.2.1. Place a PVC filter and a cellulose backup pad in each
two- or
5.2.2. Calibrate each personal sampling pump with a prepared
cassette
5.2.3. Attach prepared cassettes to calibrated sampling pumps
(the backup pad should face the pump) and place in appropriate
positions on the employee or workplace area. Collect the samples
using a total air volume of at least
5.2.4. For Time Weighted Average samples: If the filter becomes overloaded while sampling, consecutive samples using shorter sampling periods should be taken.
5.2.5. Wipe samples can be taken using PVC filters as the wipe
media. Wear clean, impervious, disposable gloves when taking each
wipe sample. If possible, wipe a surface area covering 100 cm¦. Fold
the wipe sample with the exposed side in and then transfer into a
5.2.6. If bulk samples are necessary, collect the bulk samples using a grab sampling technique suitable for the particular material(s) in use. If possible, transfer any bulk samples into 20-mL scintillation vials.
5.3. Shipment
- 5.3.1. Place plastic end caps on each cassette after sampling.
Submit at least one blank sample with each set of air samples. Blank
filter samples should be handled in the same manner as other
samples, except no air is drawn through the blank. Attach an
5.3.2. Seal scintillation vials with vinyl or electrical tape.
Securely wrap an OSHA-21
seal
5.3.3. Bulk samples should be shipped separately from air samples. They should be accompanied by Material Safety Data Sheets if available. Check current shipping restrictions and ship to the laboratory by the appropriate method.
6. Analysis
- Organic reagents should not be used.
- A water washdown system for the ducts and work surface is installed and periodically used.
- Precautions should be taken to ensure that explosions or spontaneous ignition of sample material from HClO4 is prevented.
- previously cleaned with chromic acid cleaning solution
- previously used for storage of chromium (VI) standards
- previously used for storage of bulks containing high concentrations of chromium (VI)
- After the extraction solutions are transferred to volumetric flasks and diluted to volume, place the sample beakers containing the remaining paint residue and any blanks in an exhaust hood. Carefully add 5 to 10 mL of conc. HNO3 to each beaker. Place the beakers on a hot plate and heat the samples until about 1 mL remains.
- Add 2 mL of conc. HClO4 along with a second portion of 2 mL HNO3, heat each sample, and then remove when about 1 mL remains. (Note: Please see Section 6.1.6. before using HClO4.)
- Add 1 or 2 mL of 30%
H2O2 to the
cooled solution to reduce any remaining
Cr(VI). Let the sample sit for several min and then heat for approximately 5 min to boil off the H2O2. Allow the samples to cool to room temperature. - Dilute each digested sample to a 25 mL final volume with DI H2O. Analyze these samples for chromium by atomic absorption using the procedure mentioned in reference 8.15.
- 6.1. Safety Precautions
- 6.1.1. Certain chromate compounds have been identified as
suspected carcinogens (8.10.). Care should be exercised when
handling these compounds.
6.1.2. When handling any chemicals, a labcoat, safety glasses or goggles, and gloves should be worn.
6.1.3. The buffer/extraction/electrolyte (BEE) solution is basic and somewhat corrosive. Clean up any spills immediately. This solution should be stored in polyethylene bottles since precipitated salts form readily during evaporation and will cause glass stoppers to seize. Samples prepared in glassware should be analyzed and properly disposed of as soon as possible.
6.1.4. Mercury is used as the working electrode in DPP. Always exercise caution to prevent any potential spills of mercury. Containment vessels should surround the polarograph and spill control devices should be available when handling or working with mercury.
6.1.5. Refer to the Standard Operating Procedure (SOP) (8.13.) and instrument manuals for proper operation of the polarographic instrument and safety precautions.
6.1.6. Extra care should be used when handling perchloric acid (HClO4). Perchloric acid should only be used in a hood that has been approved for HClO4 use. In this hood:
Working with HClO4 is very hazardous. Be sure to wear safety glasses, a labcoat, and gloves. Always add nitric acid (HNO3) with HClO4 when digesting samples. Watch the samples during HClO4 digestion carefully since there is a chance they could ignite. Always keep HNO3 nearby when using HClO4. In the event of sample media ignition, quickly douse the sample with a small portion of HNO3.
6.2. Equipment
- 6.2.1. Polarographic Analyzer or Controller, Model 384 or 384B,
(Princeton Applied Research, Princeton, NJ), with a Model 303 or
303A dropping mercury electrode.
6.2.2. Glass polarographic cells, 15-mL.
6.2.3. Nitrogen purification system: Gas purifier for
deoxygenating nitrogen, [(Oxiclear, part no.
6.2.4. Hot plate and exhaust hood.
6.2.5. Phillips beakers, borosilicate, 125-mL, with watch glass covers.
6.2.6. Filtration apparatus: Vacuum, vacuum flask, and PVC
filters,
6.2.7. Teflon-coated magnetic stirring bar and stirrer.
6.2.8. Micro-analytical balance (0.01 mg).
6.2.9. Polyethylene bottles, 100-mL to 1-L size.
6.2.10. Volumetric and micropipettes, volumetric flasks, beakers, and general laboratory glassware. Do not use glassware for sample analysis of chromate compounds if it was:
6.3. Reagents - All chemicals should be reagent grade or better.
- 6.3.1. Nitrogen gas.
6.3.2. Deionized water (DI H2O) with a specific conductance of less than 10 µS.
6.3.3. Sodium carbonate (Na2CO3), anhydrous.
6.3.4. Sodium bicarbonate (NaHCO3).
6.3.5. Buffer/extraction/electrolyte (BEE) solution (pH
approximately 10.5): Dissolve 100 g of
Na2CO3 and 20 g
of NaHCO3 in about 500 mL DI
H2O contained in a
6.3.6. Potassium dichromate (K2Cr2O7), or potassium chromate (K2CrO4).
6.3.7. Cr(VI) Stock Standard (1,000 µg/mL): Dissolve 2.829 g
K2Cr2O7
or 3.735 g
K2CrO4 in DI
H2O and dilute to the mark in a
6.3.8. Cr(VI) standard (100 µg/mL): Dilute 10 mL of the Cr(VI) stock standard to 100 mL with DI H2O. Prepare this solution every three months.
6.3.9. Cr(VI) working standard (10 µg/mL): Dilute 10 mL of the Cr(VI) 100 µg/mL standard to 100 mL with the BEE solution. Transfer to a polyethylene bottle. Prepare this solution daily.
6.3.10. Cr(VI) working standard (1 µg/mL): Dilute 10 mL of the Cr(VI) 10 µg/mL working standard to 100 mL with the BEE solution. Transfer to a polyethylene bottle. Prepare this solution daily.
6.3.11. Nitric acid (HNO3), concentrated (69 to 71%).
6.3.12. Nitric acid 6 M: Carefully dilute 384 mL of concentrated (conc.) HNO3 to 1 L using DI H2O.
6.3.13. Nitric acid, 10% (v/v): Carefully dilute 100 mL of conc. HNO3 to 1 L using DI H2O.
6.3.14. Perchloric acid (HClO4), conc. (69 to 71%).
6.3.15. Hydrogen peroxide (H2O2), 30%.
6.3.16. Mercury, triple distilled, for the working electrode.
6.4. Sample Preparation
- 6.4.1. Wash all glassware in hot water with detergent and rinse
with tap water, 10% HNO3, and DI
H2O (in that order). Under no
circumstances should chromic acid cleaning solutions be used.
6.4.2. Adjust the hot plate to a temperature below the boiling point of the BEE solution.
6.4.3. If bulk samples are submitted, weigh out a representative aliquot of each bulk on separate blank PVC filters.
6.4.4. Carefully remove the PVC filter from the cassette or
balance, place it
6.4.5. Allow the solutions to cool to room temperature.
Quantitatively transfer each solution to a 10- or
6.4.6. If the solution is cloudy and/or other metal analyses are desired, filter the solution through a PVC filter in a vacuum filtration apparatus. If necessary, prepare and analyze samples for other metals using the appropriate techniques. An example would be to determine the total metal content of the sample residue by atomic absorption or inductively coupled plasma spectroscopy.
6.4.7. For samples taken from spray painting operations, digest the extracted filters containing the paint residue according to the following procedure:
| Note: | Evidence indicates base extractions are capable of recovering Cr(VI) in specific paint matrices (8.4.). Due to the resistant properties of some industrial paints, an additional digestion is used for samples collected during spray painting to assure complete recovery of all Cr(VI). |
6.5. Standard Preparation
Prepare a series of Cr(VI) standards in the analytical range of 0.050 to 10 µg/mL. Make appropriate serial dilutions of the Cr(VI) working standards with the BEE solution.
6.6. Analytical Procedure
- 6.6.1. Cleaning equipment:
Soak polarographic cells in 6 M HNO3
(preferably overnight), rinse thoroughly with DI
H2O, and
6.6.2. Set the operating conditions for the instrument as follows (Note: If other types of instruments are used, refer to their operating and service manuals for comparable settings):
| Analytical technique: | DPP |
| Initial potential: | -0.100 V |
| Final potential: | -0.450 V |
| Peak potential: | -0.300 V* |
| Nitrogen purge: | 30 to 240 s |
| Scan increment: | 2 mV |
| Pulse height: | 0.050 to 0.080 V |
| Drop time: | 1 s |
| Drop size: | medium or large |
| * Varies slightly - Dependent on instrument and sample conditions | |
6.6.3. Refer to reference 8.13. or other instrument manuals for operating procedures.
6.6.4. Transfer a sample or blank to a polarographic cup. If the
final solution volume was
6.6.5. Purge each standard, blank, or sample between 30 to 240 s with purified nitrogen.
6.6.6. Analyze the reagent blank (10 mL of BEE solution), the standards, and the samples by measuring the peak current (nA). A standard should be analyzed after every five or six samples.
6.6.7. Wash the polarographic electrodes thoroughly with DI H2O after each sample is analyzed.
6.6.8. Record the peak current (nA) and potential for each
determination. A differential pulse polarogram of
6.6.9. If the peak current from a sample is above the largest standard used, an aliquot should be taken from the sample and diluted to 10 mL with BEE solution and analyzed. A dilution factor for this sample is applied when calculating results (Section 7.2.).
6.6.10. Other metals such as lead and zinc may be determined in the same solution if required. Approximate peak potentials of -0.630 V for Pb and -1.350 V for Zn were found when these species were present in the BEE solution.
7. Calculations
- 7.1. Use a least-squares regression program to plot a
7.2. Determine the air concentration of CrO3 in each extraction sample according to the following equation:
| C = | (A × SA × D × GF) - (B × SB × GF)
air volume |
| Where: | |||
| C | = | mg/m3 CrO3 | |
| A | = | amount of Cr(VI) in the sample solution (µg/mL) | |
| B | = | amount of Cr(VI) in the blank solution (µg/mL) | |
| SA | = | sample solution (mL) | |
| SB | = | blank solution (mL) | |
| D | = | dilution factor (if any) | |
| GF | = | gravimetric factor used to convert the amount of Cr(VI) to CrO3, GF = 1.923 | |
| Air Vol | = | air volume sampled (L) | |
7.3. For digested spray paint samples analyzed according to OSHA method no. ID-121, the calculations above may be used without the gravimetric factor or use calculations mentioned in that method to determine the amount of total chromium.
7.4. For bulk samples, calculate the total composition (in %) of CrO3 in each sample using:
| CrO3%(w/w) = | (A × SA)(100%) × D × GF
(sample weight)(1000 µg/mg) |
(Bulk Samples) |
Where:
- Sample wt = aliquot (in mg) of bulk taken in
Section 6.4.3.
7.5. Report air sample results (from base extractions) to the industrial hygienist as mg/m¦ CrO3.
7.6. For spray paint samples, also report results obtained from the digestion of the residue. Report each result from digested samples as mg/m¦ chromium metal and insoluble salts. Each result can be combined with the result in Section 7.5. by the industrial hygienist if the paint used during sampling does not contain other chromium compounds. Before combining results, the industrial hygienist has to perform the following calculation:
CrO3(residual) mg/m3 = Cr metal (mg/m3) + 1.923
Then:
- Total CrO3
mg/m3 =
CrO3(residual) +
CrO3(extraction)
7.7. Report bulk sample results to the industrial hygienist as approximate per cent CrO3.
8. References
- 8.1. National Institute for Occupational Safety and Health:
NIOSH Manual of Analytical Methods. 2nd ed., Vol. 1 (DHEW/NIOSH
Pub. No.
8.2. National Institute for Occupational Safety and Health:
NIOSH Manual of Analytical Methods. 2nd ed., Vol. 3 (DHEW/NIOSH
Pub. No.
8.3. Thomsen, E. and R.M. Stern: A Simple Analytical
Technique for the Determination of Hexavalent Chromium in Welding
Fumes and Other Complex Matrices. Scand. J. of Work, Environ. and
Health 5:
8.4. Molina, D. and M.T. Abell: An Ion Chromatographic
Method for Insoluble Chromates in Paint Aerosol. Am. Ind. Hyg.
Assoc. J. 48:
8.5. Dubois, L. and J.L. Monkman: Polarographic
Determination of Heavy Metals in Air Samples. Am. Ind. Hyg. Assoc.
J. 25:
8.6. Urone, P.F., M.L. Druschel and H.K. Anders:
Polarographic Microdetermination of Chromium in Dusts and Mists.
Anal. Chem. 22:
8.7. Abell, M.T. and J.R. Carlberg: A Simple Reliable Method
for the Determination of Airborne Hexavalent Chromium. Am. Ind.
Hyg. Assoc. J. 35:
8.8. Occupational Safety and Health Administration Technical
Center: Hexavalent Chromium Backup Data Report
8.9. Dutkiewicz, R., J. Konczalik and M. Przechera:
Assessment of the Colorimetric Methods of Determination of Chromium in
Air and Urine by Means of Radioisotope Techniques. Acta Pol.
Pharm. 26:
8.10. National Institute for Occupational Safety and Health:
Criteria for a Recommended Standard -- Occupational Exposure to
Cr(VI) (DHEW/NIOSH Pub. No.
8.11. National Institute for Occupational Safety and Health:
Documentation of the NIOSH Validation Tests. Backup Data
Report, Chromic Acid & Chromates, No. S317 (Contract No.
8.12. Occupational Safety and Health Administration Analytical Laboratory: Quality Control Data - Chromate Analysis by B. Babcock. Salt Lake City, UT. 1987 (unpublished).
8.13. Occupational Safety and Health Administration Technical Center: Standard Operating Procedure for Polarography. Salt Lake City, UT. In progress (unpublished).
8.14. Princeton Applied Research: Application note 108, Why Dearation... and How. Princeton, NJ: Princeton Applied Research, 1974.
8.15. Occupational Safety and Health Administration Technical
Center: Metal and Metalloid Particulates in Workplace
Atmospheres (Atomic Absorption)
8.16. Beyerman, K.: The Analytical Behavior of Minutest
Chromium Quantities, Part I. Z. Anal. Chem. 190:
8.17. Beyerman, K.: The Analytical Behavior of Minutest
Chromium Quantities, Part II. Z. Anal. Chem. 190:
Polarogram of a 1 µg/mL Cr(VI) Standard 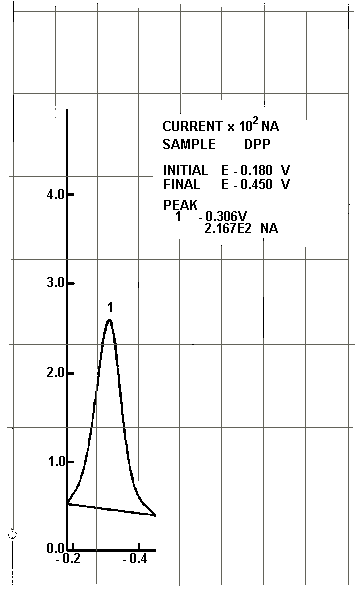
Figure 1
NA = nanoamperes