RESMETHRIN
| Method number: | 2052 |
| Matrix: | Air |
| Target Concentration: | 0.34 mg/m3 (arbitrary) There is neither an OSHA PEL nor an ACGIH TLV for resmethrin. |
| Procedure: | Samples are collected by drawing known volumes of air through
OSHA versatile sampling tubes (OVS-2) containing a glass fiber
filter and two sections of |
| Recommended air volume and sampling rate: |
60 L at 1 L/min |
| Detection limit of the overall procedure (based on the recommended air volume and the analytical detection limit): | 0.017 mg/m3 |
| Status of method: | Stopgap method. This method has been partially evaluated and is presented for information and trial use only. |
| Date: July 1989 (final draft) | Chemist: James Pike |
Carcinogen And Pesticide Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History of procedure
Recently the OSHA Analytical Laboratory received a set of field
samples with a request for the analysis of resmethrin. These air
samples had been collected on
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy).
In rats the acute oral LD50 for resmethrin (technical material) is 4240 mg/kg. In rabbits the acute dermal LD50 is 2500 mg/kg. In guinea pigs skin sensitization is negative (Ref. 5.1.).
1.1.3. Potential workplace exposure
Resmethrin is an insecticide. No estimate of the extent of worker exposure could be found at this time.
1.1.4. Physical properties and other descriptive information (Ref. 5.2. through 5.5.)
| Chemical name: | (5-Benzyl-3-fural)methyl-2, |
| Synonyms: | FMC 17370 (discontinued by FMC); NIA 17370 (discontinued by FMC) (Ref. 5.1. and 5.2.) |
| CAS #: | 10453-86-8 |
| IMIS #: | 2233 |
| Molecular weight: | 338.45 |
| Molecular formula: | C22H2603 |
| Appearance: | Waxy |
| Odor: | Characteristic chrysamthemate |
| Solubility: | Insoluble in water, 10% soluble in kerosene, very soluble in xylene, methylene chloride, isopropanol, and aromatic hydrocarbons (Ref. 5.1.) |
| UV scan: | See Figure 3. |
| Structure: | 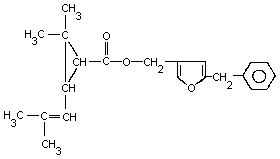 |
1.2. Limit defining parameters
The detection limit of the analytical procedure is 10.1 ng per injection. This is the amount of analyte which will give a peak whose height is approximately five times the baseline noise. (See Figure 2.)
2. Sampling Procedure (Ref. 5.6. and 5.7.)
- 2.1. Apparatus
- 2.1.1. A personal sampling pump that can be calibrated to within
± 5% of the recommended flow rate with the sampling device in line.
2.1.2.
2.2. Reagents
None
2.3. Sampling technique
- 2.3.1. Calibrate the pump to 1 L/min.
2.3.2. Attach the sampling device to the sampling pump inlet with flexible, plastic tubing so that the large, front section of the device is exposed directly to the atmosphere. Do not place any tubing in front of the sampler.
2.3.3. Attach the sampler vertically (large end down) to the worker's collar or within the breathing zone in such a manner that it does not impede work performance.
2.3.4. After sampling for the appropriate time, remove the
sampling device and reseal the tube with plastic
2.3.5. Submit at least one blank for each set of samples. Handle the blank in the same manner as the samples, except no air is drawn through it.
2.3.6. Report any possible interferences to the laboratory at the time the samples are submitted.
2.3.7. Ship samples as soon as possible in a suitable container designed to prevent damage in transit.
2.3.9. Submit bulk samples for analysis in a container separate from the air samples.
2.4. Extraction efficiency
Three "A-portions" from
Extraction Efficiency of Resmethrin
|
| ||||
| Sample | Resmethrin found | Recovery | ||
|
| ||||
| EE-5 | 32.065 µg | 79.4% | ||
| EE-6 | 33.094 mg | 81.9% | ||
| EE-7 | 32.688 mg | 80.9% | ||
| EE-8 | blank | ---- | ||
| Average recovery = 80.7% | ||||
|
| ||||
2.5. Retention efficiency
Three
Retention Efficiency of Resmethrin
|
| ||||
| Sample | Resmethrin found | Recovery | ||
|
| ||||
| RS-5 | 65.423 µg | 81.0% | ||
| RS-6 | 66.401 µg | 82.2% | ||
| RS-7 | 62.962 µg | 77.9% | ||
| RS-8 | blank | ---- | ||
| Average recovery = 80.4% | ||||
|
| ||||
2.6. Sample storage
Three
Storage Test of Resmethrin
|
| ||||
| Sample | Resmethrin found | Recovery | ||
|
| ||||
| ST-1 | 32.226 mg | 79.8% | ||
| ST-2 | 32.435 mg | 80.3% | ||
| ST-3 | 30.354 mg | 75.1% | ||
| ST-4 | blank | ---- | ||
| Average recovery = 78.4% | ||||
|
| ||||
2.7. Recommended air volume and sampling rate
- 2.7.1. The recommended air volume is 60 L.
2.7.2. The recommended flow rate is 1.0 L/min.
2.8. Interferences (sampling)
It is not known if any compounds will interfere with the collection of resmethrin. Suspected interferences should be reported to the laboratory.
2.9. Safety precautions (sampling)
- 2.9.1. Attach the sampling equipment in such a manner that it
will not interfere with work performance or employee safety.
2.9.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure (Ref. 5.7.)
- 3.1. Apparatus
- 3.1.1. A calibrated balance capable of determining a weight to
the nearest 100 µg. A Mettler HL52 balance was used in this
evaluation to prepare the concentrated standards.
3.1.2. Volumetric flasks, pipets, and syringes of various convenient sizes for preparing standards, making dilutions and making injections.
3.1.3. A high performance liquid chromatograph equipped with a
variable wavelength UV detector, manual or automatic injector, and
strip chart recorder. A
3.1.4. An HPLC column capable of separating resmethrin from any interferences. A (25 cm × 4.6 mm i.d.) Du Pont Zorbax ODS (5 micron) column was used for this evaluation.
3.1.5. An electronic integrator, or some other suitable method
for measuring detector response. The
3.1.6. Glass vials,
3.1.7. Glass vials,
3.2. Reagents
- 3.2.1. Acetonitrile (AcN), HPLC grade
3.2.2. Water, HPLC grade. A Millipore
3.2.5. Resmethrin, (EPA Reference standard #6055). A 99.5% pure standard from EPA was used for this evaluation.
3.3. Standard preparation
Prepare two stock standard solutions by adding acetonitrile to
preweighed amounts of resmethrin (20 to 30 mg) in
3.4. Sample preparation
- 3.4.1. Transfer the glass fiber filter and the
3.4.2. Add 2.0 ml of acetonitrile to each vial and seal with rubber lined caps.
3.4.3. Allow the samples to extract for one hour with occasional vigorous shaking.
3.4.4. Where necessary for automation, transfer aliquots of
samples to
3.5. Analysis
- 3.5.1. Instrument conditions
| Column: | Du Pont Zorbax ODS, |
| Column temperature: | 50°C |
| Mobile phase: | AcN/H2O 75:25 |
| Mobile phase temperature: | 50°C |
| Flow rate: | 1.5 mL/min |
| Detector wavelength: | 233 nm |
| Retention time: | 5.7 min |
| Injection volume: | 20 µL |
3.5.2. Chromatogram (See Figure 1.)
3.5.3. Intersperse the analytical standards, including a detection limit standard, with the samples in the analytical sequence.
3.6. Interferences (analytical)
- 3.6.1. Any collected compound soluble in acetonitrile that has a
similar retention time and absorbs at 233 nm is an interference.
Generally, chromatographic conditions can be altered to separate
interferences from the analyte.
3.6.2. Retention time alone is not proof of chemical identity. Confirmation of chemical identity by other means such as GC Mass Spectrometry, detector response ratioing or chromatography by an alternate column should be sought when possible.
3.7. Calculations
- 3.7.1. By using a suitable method, such electronic integration,
measure the detector response.
3.7.2. Use an external or internal standard procedure to prepare a calibration curve using analytical standards over a range of concentrations (See Figure 4). Bracket the samples with analytical standards.
3.7.3. Construct a calibration curve by plotting detector response versus standard concentration. (See Figure 4.)
3.7.4. Determine the concentration of resmethrin in each section of a sample from the calibration curve. If resmethrin is found on the backup section, add it to the amount on the front section. Make blank corrections for each section before adding the results together.
3.7.5. The air concentration is then determined by:
| mg/m3 = | (µg/mL in sample) × (extraction
volume, mL)
(air volume, L) × (extraction efficiency, decimal) |
3.8. Safety precautions (analytical)
- 3.8.1. Avoid exposure to all standards.
3.8.2. Avoid skin contact with all solvents.
3.8.3. Wear safety glasses in the laboratory at all times.
4. Recommendation for Further Study
A fully validated sampling and analytical method should be developed.
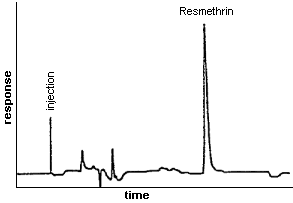
Figure 1. Chromatogram of Resmethrin 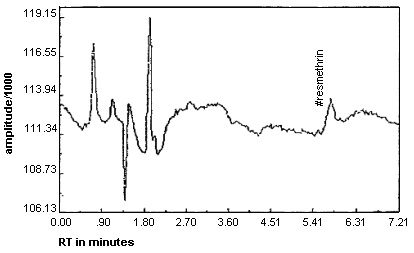
Figure 2. Detection limit of Analytical Procedure for Resmethrin
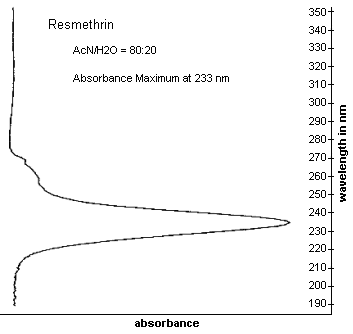
Figure 3. UV Scan of Resmethrin 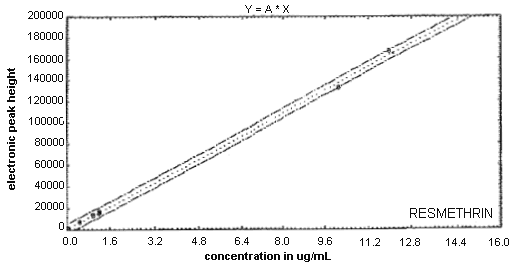
Figure 4. Calibration Curve of Resmethrin 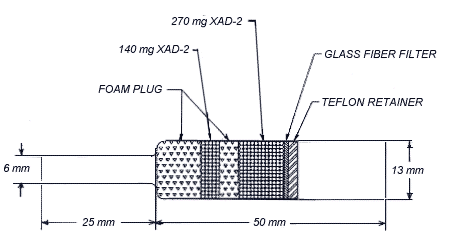
Figure 5.
5. References
- 5.1. Meister, R. T., Ed.; "Farm Chemicals Handbook '85", 71st ed.;
Meister Publishing: Willowby, OH, 1985; p C203.
5.2 Ouellett, R. P. and King, J. A.; "Chemical Week Pesticides
Register";
5.3. Wiswesser, W. J., Ed.; "Pesticide Index", 5th ed.; Entomological Society of America: College Park, MD, 1976; p 198.
5.4. Watts, R. R., Ed.; "Analytical Reference Standards and Supplemental Data for Pesticides and Other Organic Compounds"; Analyt ical Chemistry Branch, Environmental Toxicology Division, Health Effects Research Laboratory: Research Triangle Park, NC, Revised 1980; p 85.
5.5. Tatken, R. L. and Lewis, R. J. Sr., Ed.; "Registry of Toxic
Effects of Chemical Substances"; U. S. Department of Health and Human
Services: Cincinnati, OH, 1986; DDH(NIOSH) Publication No.
5.6. "Industrial Hygiene Technical Manual"; OSHA Instruction CPL
5.7 Burright, D.; "Carbaryl (Sevin)"; Method #58, Organic Methods Evaluations Branch, OSHA Analytical Laboratory: Salt Lake City, UT, July 1987; unpublished.