1,5-NAPHTHALENE DIISOCYANATE (NDI)
| Method number: | PV2046 |
| Matrix: | Air |
| Target Concentration: | 0.02 ppm (170 µg/m3) (arbitrary). There is no OSHA permissible exposure level (PEL) or ACGIH threshold limit value (TLV) for NDI. |
| Procedure: | Samples are collected by drawing a known volume of air through a
glass fiber filter (GFF) coated with 1.0 mg of
|
| Recommended air volume and sampling rate: |
60 L at 1.0 L/min |
| Detection limit of the overall procedure (based on the recommended air volume and the analytical detection limit): |
8.0 µg/m3 |
| Special precautions: | The |
| Status of method: | Partially validated method. This method is presented for information and trial use only. |
| Date: December 1993 (final) | Chemist: Ing-Fong Chan |
Organic Service Branch II
OSHA Salt Lake Technical
Center
Salt Lake City, UT 84165-0200
1. General Discussion
- 1.1. Background
- 1.1.1. History of procedure
This evaluation was undertaken to determine the effectiveness of
a
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Inhalation of NDI vapors or mists can cause irritation of the mucous membranes in the respiratory tract, running nose, sore throat, productive cough, shortness of breath or asthma, as has been reported with other isocyanates (eg. TDI and MDI) (Ref. 5.2.).
From the available information on NDI it appears that its toxicological action is similar to other isocyanates (eg. TDI and MDI). For this reason the target concen-tration adopted 0.02 ppm, the same as MDI. (Ref. 5.1.).
1.1.3. Potential workplace exposure
NDI is used in making high grade urethane cast elastomers (Ref. 5.2.). No information was available on the number of workers potentially exposed to NDI.
1.1.4. Physical properties (Ref. 5.2.)
| Chemical name: | naphthalene, 1,5-diisocyanato- |
| Synonyms name: | 1,5-naphthalene diisocyanate (NDI) |
| CAS number: | 3173-72-6 |
| Molecular formula: | C12H6N2O2 |
| Structural formula: | 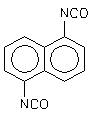 |
| Molecular weight: | 210.2 |
| Boiling point: | 244 °C at 13.3 kPa (100 mmHg) |
| Melting point: | 127 °C |
| Specific gravity: | 1.45 at 20 °C |
| Solubility: | Soluble in water. |
| Appearance: | White to pale yellow flakes |
1.2. Limit defining parameters
- 1.2.1. The detection limit of the analytical procedure (based on
fluorescence detector)
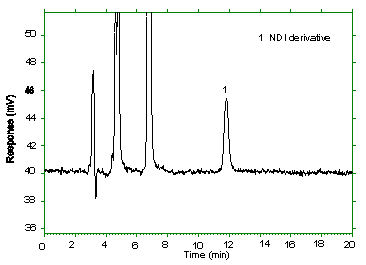
The detection limit of the analytical procedure is 1.63 ng as NDI
per injection. Usually this is the amount of analyte which gives a
peak whose height is approximately five times the baseline noise.
Because of the interfence from
1.2.2. Detection limit of the overall procedure (based on fluorescence detector)
The detection limit of the overall procedure is 0.49 µg as NDI per sample. This is the amount of analyte spiked on a glass fiber filter that, upon analysis, produces a peak similar in size to that of the detection limit of the analytical procedure.

2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Samples are collected using a personal sampling pump that
can be calibrated within ±5% of the recommended flow rate with the
sampling device attached.
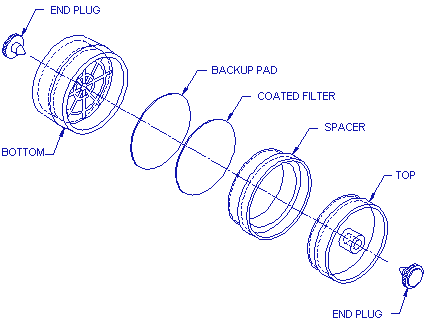
2.1.2. A three-piece polystyrene cassette containing a glass
fiber filter coated with 1.0 mg of
2.2. Glass fiber filter coating procedure
Prepare a solution of 2 mg/mL of
Store coated glass fiber filters in a closed jar at reduced
temperature as a precaution to prevent decomposition of the
2.3. Sampling technique
- 2.3.1. Open-face sampling is performed by removing the top cover
from the
2.3.2. Attach the sampler to the sampling pump with a piece of flexible tubing and place it in the worker's breathing zone.
2.3.3. After sampling for the appropriate time, remove the tube and reseal the cassette with the plastic plugs. Wrap each sample end-to-end with an OSHA seal (Form 21).
2.3.4. Record the air volume for each sample and list any possible interferences.
2.3.5. Submit at least one blank for each set of samples. The blank is handled in the same manner as the samples except no air is drawn through it.
2.3.6. Submit bulk samples for analysis in a separate container from the air samples.
2.4. Extraction efficiency
Three groups of four
Extraction Efficiency of NDI
at 0.5× Target Concentration
|
| |||
| sample i.d. |
µg spiked |
µg recovered |
recovery (decimal) |
|
| |||
| D1 D2 D3 D4 |
4.875 4.875 4.875 4.875 |
4.623 4.541 4.638 4.609 |
0.948 0.931 0.951 0.945 |
|
| |||
Extraction Efficiency of NDI
at 1× Target Concentration
|
| |||
| sample i.d. |
µg spiked |
µg recovered |
recovery (decimal) |
|
| |||
| D5 D6 D7 D8 |
9.750 9.750 9.750 9.750 |
9.582 9.497 9.524 9.443 |
0.983 0.974 0.977 0.969 |
|
| |||
Extraction Efficiency of NDI
at 2× Target Concentration
|
| |||
| sample i.d. |
µg spiked |
µg recovered |
recovery (decimal) |
|
| |||
| D9 D10 D11 D12 |
19.50 19.50 19.50 19.50 |
18.99 19.26 19.07 19.27 |
0.974 0.988 0.978 0.988 |
|
| |||
2.5. Retention efficiency
Five ![]() 80%
relative humidity) were drawn through each of the samples at 1.0
L/min. The samples were analyzed as per section 3. The results are
listed in Table 2.5. The average retention efficiency from the five
samplers was 0.978.
80%
relative humidity) were drawn through each of the samples at 1.0
L/min. The samples were analyzed as per section 3. The results are
listed in Table 2.5. The average retention efficiency from the five
samplers was 0.978.
Retention Efficiency of NDI
at 1× Target Concentration
|
| |||
| sample i.d. |
µg spiked |
µg recovered |
recovery (decimal) |
|
| |||
| RE-1 RE-2 RE-3 RE-4 RE-5 |
9.750 9.750 9.750 9.750 9.750 |
9.424 9.693 9.583 9.477 9.473 |
0.967 0.994 0.983 0.972 0.972 |
|
| |||
2.6. Sample storage
Ten ![]() 80%
relative humidity) were drawn through each of the sample cassettes at
1.0 L/min. The ten samples were divided into two groups of five
cassettes each. The first group was stored in a refrigerator (0°C),
the second group was stored in a drawer at ambient temperature. After
eight days they were extracted and analyzed as in Section 3. The
results are listed in Table 2.6.1. and 2.6.2. The average recoveries
for the refrigerator and ambient temperature storage studies were
0.974 and 0.954, respectively.
80%
relative humidity) were drawn through each of the sample cassettes at
1.0 L/min. The ten samples were divided into two groups of five
cassettes each. The first group was stored in a refrigerator (0°C),
the second group was stored in a drawer at ambient temperature. After
eight days they were extracted and analyzed as in Section 3. The
results are listed in Table 2.6.1. and 2.6.2. The average recoveries
for the refrigerator and ambient temperature storage studies were
0.974 and 0.954, respectively.
Refrigerator Storage
|
| |||
| Days of Storage |
µg Spiked |
µg recovered |
recovery (decimal) |
|
| |||
| 8 8 8 8 8 |
9.750 9.750 9.750 9.750 9.750 |
9.640 9.639 9.420 9.465 9.288 |
0.989 0.989 0.966 0.971 0.953 |
|
| |||
Ambient Storage
|
| |||
| Days of Storage |
µg Spiked |
µg recovered |
recovery (decimal) |
|
| |||
| 8 8 8 8 8 |
9.750 9.750 9.750 9.750 9.750 |
9.385 9.348 9.205 9.273 9.289 |
0.963 0.959 0.944 0.951 0.953 |
|
| |||
2.7. Recommended air volume and sampling rate
- 2.7.1. The recommended air volume is 60 L.
2.7.2. The recommended flow rate is 1.0 L/min.
2.8. Interferences (sampling)
It is not known if any compounds will interfere with the collection of NDI. Any suspected interferences should be reported to the laboratory.
2.9. Safety precautions (sampling)
- 2.9.1. Attach the sampling equipment in such a manner that it
will not interfere with work performance or employee safety.
2.9.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A balance capable of weighing to the nearest one
hundredth of a milligram. A Mettler AE240 balance was used in this
evaluation to prepare the concentrated standards.
3.1.2. Volumetric flasks, pipets, and syringes of various convenient sizes for preparing standards, making dilutions and making injections.
3.1.3. Glass vials, 4-mL with PTFE-lined caps.
3.1.4. An HPLC equipped with a UV detector, and a WISP auto-sampler were used in this evaluation.
3.1.5. An HPLC column capable of separating NDI from any interferences. An Econosphere CN column (4.6 × 250 mm) was used in this evaluation.
3.1.6. An electronic integrator, or some other suitable means for measuring detector response. The Waters 860 Data System was used in this evaluation.
3.2. Reagents
- 3.2.1. Acetonitrile, HPLC grade. The solvent used in this
evaluation was obtained from Burdick and Jackson, Baxter Healthcare
Co.
3.2.2. Dimethyl sulfoxide, HPLC grade. The solvent used in this evaluation was obtained from Burdick and Jackson, Baxter Healthcare Co.
3.2.3. Water, HPLC grade. A Millipore Milli-Q system was used to prepare the water in this evaluation.
3.2.4. 1-(2-pyridyl)piperazine.
3.2.5. NDI. NDI, 100% purity, was obtained from Bayer USA INC.
3.2.6. Extraction solution: 90/10 (V/V) ACN/DMSO.
3.3. Standard preparation
- 3.3.1. Prepare stock standards by weighing 10 mg of NDI in 10-mL
volumetric flasks and diluting to volume with DMSO containing excess
3.3.2. Prepare analytical standard by diluting the stock standards with 90/10 (V/V) ACN/DMSO. A 3.4 µg/mL standard solution corresponds to the target concentration.
3.3.3. Prepare a sufficient number of analytical standards to generate a calibration curve. Analytical standard concentrations must bracket sample concentrations.
3.4. Sample preparation
- 3.4.1. Transfer the
3.4.2. Add 3.0 mL of extracting solution to each vial.
3.4.3. Cap the vials and shake them on a mechanical shaker for 30 min.
3.5. Analysis
- 3.5.1. Instrument conditions
| Column: | Econosphere CN |
| Eluent: | 42.5/57.5 (V/V) ACN/Water ,0.075M Ammonium acetate, pH 5.9 |
| Flow rate: | 1.0 mL/min |
| Injection volume: | 10 µL |
| Retention time: | 11.8 min |
| UV detector: | 254 nm |
| Fluororescence detector: | excitation 240 nm, emission 370 nm |
| Chromatogram: | based on fluorescence |

3.6. Interferences (analytical)
- 3.6.1. Any compound that responds to fluorescence or UV detector
under the analytical conditions and has a similar retention time as
the analyte is a potential interference. Generally, chromatographic
conditions can be altered to separate an interference from the
analyte.
3.6.2. Retention time on a single column is not considered proof of chemical identity. Additional means of identification include: using an alternate HPLC column, or detectionat another wavelength (peak ratioing) or detector ratioing (fluorescene/UV).
3.7. Calculations
- 3.7.1. Construct a calibration curve by plotting detector
response versus concentration (µg/mL) of NDI.
3.7.2. Determine the µg/mL of NDI in samples and blank from the calibration curve.
3.7.3. Blank correct each sample by subtracting the µg/mL found in the blank from the µg/mL found in the corresponding samples.
3.7.4. Determine the air concentration by using the following formulae.
| mg/m3 = | (µg/mL, blank corrected) ×
(desorption volume, mL)
(air volume, L) × (desorption efficiency, decimal) |
| ppm = | (mg/m3)(24.46)
210.2 |
| where | 24.46 | = | molar volume (liters) at 101.3 kPa (760 mmHg) and 25°C |
| 210.2 | = | molecular weight of NDI |
3.8. Safety precautions (analytical)
- 3.8.1. Avoid skin contact and air exposure to NDI.
3.8.2. Avoid skin contact and air exposure to all solvents.
3.8.3. Wear safety glasses at all times in the laboratory.
4. Recommendation for Further Study
This method needs to be fully validated and try to make a purified standard..
5. References
- 5.1. "OSHA Analytical Methods Manual", U.S. Department of Labor,
Occupational Safety and Health Administration, OSHA Analytical
Laboratory: Salt Lake City, UT, Method 47, American Conference of
Governmental Industrial Hygienists (ACGIH): Cincinnati, OH, 1985,
ISBN: 0-936712-66-x.
5.2. "Material Safety Data Sheet", Mobay corporation, polyurethane division, Bayer USA Inc. 1993