MONOCROTOPHOS
| Method number: | PV2045 |
| Matrix: | Air |
| Target Concentration: | 0.25 mg/m3 OSHA permissible exposure level (PEL). |
| Procedure: | Samples are collected by drawing known volumes of air through OSHA versatile sampler (OVS-2) tubes, each containing a glass fiber filter and two sections of XAD-2 adsorbent. Samples are desorbed with toluene and analyzed by gas chromatography (GC) using a flame photometeric detector (FPD). |
| Recommended air volume and sampling rate: |
480 L at 1.0 L/min |
| Detection limit of the overall procedure (based on the recommended air volume and the analytical detection limit): |
7.4 µg/m3 |
| Status of method: | Stopgap method. This method has been partially evaluated and is presented for information and trial use only. |
| Date: May 1990 (final) | Chemist: Duane Lee |
Carcinogen And Pesticide Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History of procedure
This evaluation was undertaken because OSHA recently adopted the TLV's as PEL's. The OVS-2 sampler was tested as an effective sampling device for monocrotophos. This method follows the procedures developed for other organophosphorus pesticides. (Ref. 5.1.)
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Monocrotophos is an organophosphorus pesticide. It has an oral LD50 of 17 mg/kg in male rats and 20 mg/kg in female rats. (Ref. 5.4.) It is a highly toxic, direct acting, cholinesterase inhibitor, which appears to be capable of penetration through the intact skin; but is excreted rapidly and does not appear to accumulate within the body (Ref. 5.5.).
1.1.3. Potential workplace exposure
There was no information available on the number of workers exposed to monocrotophos which is used as a systemic insecticide. There was an estimated 2.4×109 grams of monocrotophos produced in the U.S. in 1974. (Ref. 5.6.)
1.1.4. Physical properties (Ref. 5.2. to 5.5.)
| CAS number: | 6923-22-4 |
| IMIS number: | 2690 |
| Molecular weight: | 233.16 |
| Molecular formula: | C7H14NO5P |
| Melting point: | 54-55°C |
| Boiling point: | 125°C |
| Solubility: | soluble in ethanol, acetone, and water; practically insoluble in diesel oils and kerosene |
| Chemical name: | dimethyl 2-methylcarbamoyl-1-methyl-vinyl phosphate |
| Synonyms: | Apadrin, Azodrin, Biloborn, C1414, Crisodrin, Hazodrin, SO9129, Monocron, Nuvacron, Plantdrin, Pilladrin |
| Description: | reddish brown solid with a mild ester odor |
| Structure: | 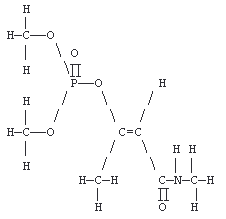 |
1.2. Limit defining parameters
The detection limit of the analytical procedure, including a 28:1 split ratio, is 0.063 ng per injection. This is the amount of analyte which will give a peak whose height is approximately five times the baseline noise. (Figure 1.)
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. A personal sampling pump that can be calibrated to within
±5% of the recommended flow rate with the sampling device in line.
2.1.2. OVS-2 tubes, which are specially made 13-mm o.d. glass tubes that are tapered to 6-mm o.d., packed with two sections of cleaned XAD-2 adsorbent and a 13-mm diameter glass fiber filter. The sampling section and backup section contain 270 and 140 mg adsorbent respectively. The backup section is retained by two foam plugs, and the sampling section is between a foam plug and the glass fiber filter. The glass fiber filter is held next to the sampling section by a polytetrafluoroethylene (PTFE) retainer. (Figure 2.)
2.2. Reagents
No sampling reagents are required.
2.3. Sampling technique
- 2.3.1. Immediately before sampling, remove the plastic caps from
the OVS-2 tube.
2.3.2. Attach the small end of the tube to the sampling pump with flexible tubing.
2.3.3. Attach the tube vertically in the employee's breathing zone in such a manner that it does not impede work performance.
2.3.4. After sampling for the appropriate time, remove the tube and seal it with plastic caps.
2.3.5. Wrap each sample end-to-end with an OSHA seal (Form 21).
2.3.6. Record the air volume for each sample, and list any possible interferences.
2.3.7. Submit at least one blank for each set of samples. Handle the blank in the same manner as the samples, except no air is drawn through it.
2.3.8. Submit bulk samples for analysis in a separate container. Do not ship with air samples.
2.4. Desorption efficiency (glass fiber filter and XAD-2 adsorbent)
Six vials each containing a 13-mm glass fiber filter and 270-mg of XAD-2 adsorbent were each liquid spiked on the glass fiber filter with 8.2 µL of a 14.7 mg/mL standard of monocrotophos and allowed to dry overnight in a drawer at ambient temperature. These samples were each desorbed with 2.0 mL of toluene containing triphenyl phosphate (TPP) as the internal standard, shaken for 30 min and analyzed as in Section 3. The results are listed in Table 2.4.
Desorption Efficiency
| Amount | Amount | % | |
| Sample # | Spiked, µg | Found, µg | Recovered |
| Ex1 Ex2 Ex3 Ex4 Ex5 Ex6 |
120.54 120.54 120.54 120.54 120.54 120.54 |
112.66 117.83 107.09 118.76 117.59 116.49 |
93.5 97.7 88.8 98.5 97.5 96.6 |
| Average = 95.4 | |||
2.5. Retention efficiency
Eighteen OVS-2 tubes were each liquid spiked with 8.2 µL of a 14.7 mg/mL monocrotophos standard on the glass fiber filter. These were allowed to dry overnight and then 480 L of humid air (~77% relative humidity) were drawn through each tube at 1 L/min. Six of the tubes were each desorbed with 2.0 mL of toluene containing TPP, shaken for 30 min and then analyzed as in Section 3. The results are listed in Table 2.5. The remaining samples were stored, 6 in a drawer at ambient temperature and 6 in a freezer.
Retention Efficiency
| Amount | Amount | % | |
| Sample # | Spiked, µg | Found, µg | Recovered |
| R1 R2 R3 R4 R5 R6 |
120.54 120.54 120.54 120.54 120.54 120.54 |
99.75 110.41 96.83 96.06 96.67 106.7 |
82.8 91.6 80.3 79.7 80.2 88.5 |
| Average = 83.8 | |||
2.6. Sample storage
After 3 days of storage, 6 tubes, 3 from the ambient storage group and 3 from the freezer storage group, were each desorbed with 2.0 mL of toluene containing TPP, shaken for 30 min and then analyzed as in Section 3. The remaining tubes were desorbed and analyzed after 10 days of storage. The results are given in Tables 2.6.1. and 2.6.2.
Ambient Storage
| Amount | Amount | % | |
| Sample # | Spiked, µg | Found, µg | Recovered |
| 3 3 4 9 10 10 |
120.54 120.54 120.54 120.54 120.54 120.54 |
99.8 100.59 93.2 92.42 108.03 99.43 |
82.8 83.4 77.3 76.7 89.6 82.5 |
| Average of ~3 days = 81.2 | |||
| Average of ~10 days = 82.9 | |||
Freezer Storage
| Amount | Amount | % | |
| Sample # | Spiked, µg | Found, µg | Recovered |
| 3 3 3 9 9 9 |
120.54 120.54 120.54 120.54 120.54 120.54 |
101.16 104.47 104.09 102.14 104.0 105.14 |
83.9 86.7 86.4 84.7 86.3 87.2 |
| Average of 3 days = 85.7 | |||
| Average of 9 days = 86.1 | |||
2.7. Recommended air volume and sampling rate
- 2.7.1. The recommended air volume is 480 L.
2.7.2. The recommended flow rate is 1.0 L/min.
2.8. Interferences (sampling)
It is not known if any compounds will interfere with the collection of monocrotophos. Any suspected interferences should be reported to the laboratory.
2.9. Safety precautions (sampling)
- 2.9.1. Attach the sampling equipment in such a manner that it
will not interfere with work performance or employee safety.
2.9.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A balance capable of weighing to the nearest tenth of a
milligram. A Mettler HL52 balance was used in this evaluation.
3.1.2. A mechanical shaker.
3.1.3. A GC equipped with an FPD. A Hewlett Packard (HP) 5890 equipped with an autosampler was used in this evaluation.
3.1.4. A GC column capable of separating monocrotophos from any interferences. A 30 m × 0.32 mm i.d. (1.0 µm film) DB-5 capillary column was used in this evaluation.
3.1.5. An electronic integrator, or some other suitable means for measuring detector response. A Waters 860 Laboratory Data System was used in this evaluation.
3.1.6. Volumetric flasks and pipets.
3.1.7. Vials, 2-mL.
3.2. Reagents
- 3.2.1. Toluene, reagent grade.
3.2.2. Monocrotophos, reagent grade. A standard obtained from EPA (EPA # 0360, 98% purity) was used in this evaluation.
3.2.3. Triphenyl phosphate (TPP), reagent grade. A 40 µg/mL solution in toluene was used as an internal standard.
3.3. Standard preparation
Prepare monocrotophos stock standards by weighing 10 to 15 mg of monocrotophos. Transfer the monocrotophos to separate 10-mL volumetric flasks, and add toluene to the mark. Make working range standards of 1.7 to 147 µg/mL by pipet dilutions of the stock standards with toluene containing TPP. Store stock and dilute standards in a freezer.
3.4. Sample preparation
- 3.4.1. Transfer the 13-mm glass fiber filter and the 270-mg
sampling section of the tube to a
3.4.2. Add 2.0 mL of toluene containing TPP to each vial and seal with a Teflon-lined cap.
3.4.3. Shake the vials for 30 minutes on a mechanical shaker.
3.4.4. Transfer, if necessary, the samples to 2-mL vials for use on an HP autosampler.
3.5. Analysis
- 3.5.1. Instrument conditions
| Column: | DB-5, 30 m × 0.32 mm i.d., 1.0 µm film |
| Injector temperature: | 250°C |
| Column temperature: | 220°C |
| Detector temperature: | 225°C |
| Gas flows: | |
| Column: | 1 mL/min hydrogen |
| FPD make up: | 42 mL/min nitrogen |
| Injection volume: | 1 µL |
| Split ratio: | 28:1 |
| Retention time: | 4.66 min |
3.5.2. Chromatogram (Figure 3.)
3.6. Interferences (analytical)
- 3.6.1. Any collected compound having a similar retention time to
that of the analyte is a potential interference.
3.6.2. GC conditions may generally be varied to circumvent interferences.
3.6.3. Retention time on a single column is not proof of chemical identity. Analysis by an alternate GC column, high performance liquid chromatography (HPLC) and confirmation by mass spectrometry are additional means of identification.
3.7. Calculations
- 3.7.1. Construct a calibration curve (Figure 4.) by plotting
detector response versus concentration (µg/mL) of monocrotophos.
3.7.2. Determine the µg/mL of monocrotophos in both sections of each sample and blank from the calibration curve.
3.7.3. Blank correct each section by subtracting the µg/mL found in each blank section from the µg/mL found in each corresponding sample section and then add the values together.
3.7.4. Determine the air concentration by using the following formula.
| mg/m3 = | (µg/mL, blank corrected) ×
(desorption volume, mL)
(air volume, L) × (desorption efficiency, decimal) |
3.8. Safety precautions (analytical)
- 3.8.1. Avoid skin contact and air exposure to monocrotophos.
3.8.2. Avoid skin contact with all solvents.
3.8.3. Wear safety glasses at all times.
4. Recommendation for Further Study
This method should be fully validated.
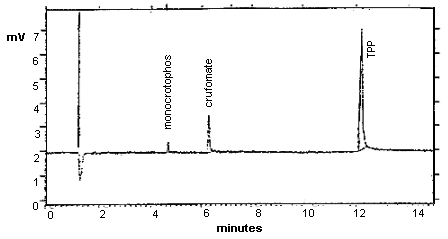
Detection Limit Chromatogram for Monocrotophos with Crufomate and TPP
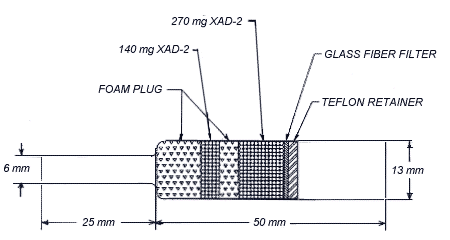
OVS-2 Sampling Tube
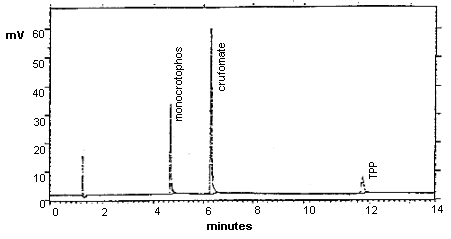
Chromatogram of Monocrotophos with Crufomate and TPP

Calibration Curve
5. References
- 5.1. Burright, D., Method #62, Chlorpyrifos, DDVP, Diazinon,
Malathion, and Parathion, OSHA Analytical Laboratory, unpublished,
1986.
5.2. Registry of Toxic Effects of Chemical Substances 1985-86 Edition; DHHS(NIOSH) Publication No. 87-114, U.S. Department of Health and Human Services: Cincinnati, OH, 1987; p 3392.
5.3. Farm Chemicals Handbook; Berg, Gordon L. Ed.; Meister: Willoughby, Ohio, 1989; p C201.
5.4. Merck Index, 10th ed.; Windholz, Martha ED.; Merck: Rahway, N.J., 1983; p 894.
5.5. Documentation of Threshold Limit Values and Biological Exposure Indices; American Conference of Governmental Industrial Hygienists Inc., Fifth Edition, 1986, p 416.
5.6. HSDB (Hazardous Substance Data Base).