METHYL BROMIDE
| Method number: | PV2040 |
| Matrix: | Air |
| Target concentration: | 20 ppm (80 mg/m3) OSHA ceiling
(skin) 5 ppm (20 mg/m3) ACGIH TWA (skin) |
| Procedure: | Samples are collected by drawing a known volume of air through
two Anasorb 747 tubes (400/200 mg) connected in series. Samples are
separated after sampling, caps are placed on tube ends, and each
sample is sealed separately. Samples are desorbed with 2 mL of
carbon disulfide, while the vials are in an ice filled tray. Samples
are shaken for 30 minutes and analyzed by gas chromatography using a
flame ionization detector |
| Recommended air volume and sampling rate: |
Maximum air volume = 3 L Maximum flow rate = 0.2 L/min (15 minute ceiling sample) Minimum flow rate = 0.05 L/min |
| Reliable quantitation limit: | 0.19 ppm (0.74 mg/m3) |
| Special requirements: | Samples must be refrigerated immediately after sampling, shipped with ice or synthetic ice product, and analyzed within two weeks of sampling. |
| Status of method: | Partially Evaluated Method. This method has been subjected to established evaluation procedures, and is presented for information and trial use. |
| Date: April 1995 | Chemist: Mary E. Eide |
Organic Service Branch I
OSHA Salt Lake Technical
Center
Salt Lake City, UT 84165-0200
1. General Discussion
- 1.1 Background
- 1.1.1 History
The NIOSH method 2520 (Ref. 5.1) for methyl bromide uses two
charcoal tubes in series for collection. In a field study conducted
in 1990, following this method for sampling and analysis, they found
that the methyl bromide had a capacity problem and poor storage
(Ref. 5.2). The capacity of the charcoal tubes was dependant on the
humidity of the air stream being sampled, at 40% RH breakthrough was
at 12.2 L, but at 80% RH breakthrough was at 2.3 L. Storage studies
showed only 60% recovered after 8 days at
1.1.2 Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.) (Ref. 5.3)
Exposure of 90 workers to 35 ppm methyl bromide for two weeks produced the following symptoms in the workers: nausea, vomiting, headache, skin lesions, and symptoms of mild systemic poisoning. Symptoms of acute poisoning, exposures above 200 ppm, are muscle weakness and pain, loss of coordination and gait, inability to focus eyes, pulmonary edema, convulsions, hyperthermia, and coma. Prolonged exposures at this level results in death, usually resulting from irreversible pulmonary edema.
1.1.3 Workplace exposure (Ref.5.3 and 5.4)
Methyl bromide is used as a fumigant for soil and foodstuffs, for termite control, as a nematocide, in ionization chambers, to degrease wool, as a selective solvent in aniline dyes, as a methylating agent, and to extract oil from flowers, nuts, and seeds. Methyl bromide was once used as a refrigerant and in fire extinguishers, but these uses have been discontinued due to serious injuries and/or deaths.
1.1.4 Physical properties and other descriptive information (Ref. 5.3 and 5.4)
| Synonyms: | Bromomethane; Dowfume; Embafume;
Halon 1001; Metafume; Monobromomethane; Pestmaster; Profume;
|
| CAS number: | 74-83-9 |
| IMIS: | 1680 |
| RTECS: | PA4900000 |
| DOT: | UN1062 (poison gas) |
| Molecular weight: | 94.95 |
| Boiling point: | 3.56°C |
| Melting point: | -93.66°C |
| Odor: | odorless except in high concentrations, then a sweetish, chloroform-like odor |
| Color: | colorless gas |
| Molecular formula: | CH3Br |
| Structural formula: | 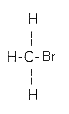 |
The analyte air concentrations throughout this method are based on the recommended sampling and analytical parameters. Air concentrations listed in ppm are referenced to 25°C and 101.3 kPa (760 mmHa).
- 1.2 Limit defining parameters
- 1.2.1 Detection limit of the overall procedure (DLOP)
The detection limit of the overall procedure is 0.664 µg per sample (0.056 ppm or 0.221 mg/m3). This is the amount of analyte spiked on the sampler that will give a response that is significantly different from the background response of a sampler blank.
The DLOP is defined as the concentration of analyte that gives a response (YDLOP) that is significantly different (three standard deviations (SDBR)) from the background response (YBR).
The direct measurement of YBR and SDBR in chromatographic methods is typically inconvenient, and difficult because YBR is usually extremely low. Estimates of these parameters can be made with data obtained from the analysis of a series of samples whose responses are in the vicinity of the background response. The regression curve obtained for a plot of instrument response versus concentration of analyte will usually be linear. Assuming SDBR and the precision of data about the curve are similar, the standard error of estimate (SEE) for the regression curve can be substituted for SDBR in the above equation. The following calculations derive a formula for the DLOP:
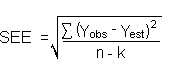
| Yobs | = | observed response |
| Yest | = | estimated response from regression curve |
| n | = | total no. of data points |
| k | = | 2 for a linear regression curve |
At point YDLOP on the regression curve
| YDLOP = A(DLOP) + YBR | A = analytical sensitivity (slope) |
therefore
| DLOP = | (YDLOP -
YBR)
A |
Substituting 3(SEE) + YBR for YDLOP gives
| DLOP = | 3(SEE)
A |
The DLOP is measured as mass per sample and expressed as equivalent air concentrations, based on the recommended sampling parameters. Ten samplers were spiked with equal descending increments of analyte, such that the lowest sampler loading was 3 µg/sample. This is the amount, when spiked on a sampler, that would produce a peak approximately 10 times the background response for the sample blank. These spiked samplers, and the sample blank, were analyzed with the recommended analytical parameters, and the data obtained used to calculate the required parameters (A and SEE) for the calculation of the DLOP. Values of 154 and 34.1 were obtained for A and SEE respectively. The DLOP was calculated to be 0.664 µg/sample (0.114 ppm or 0.443 mg/m3).
| Table 1.2.1 Detection Limit of the Overall Procedure | |
| mass per sample | area counts |
| (µg) | (µV-s) |
|
| |
| 0 3 6 9 12 15 18 21 24 27 30 |
0 269 472 741 947 1219 1400 1630 1816 2105 2394 |
|
| |
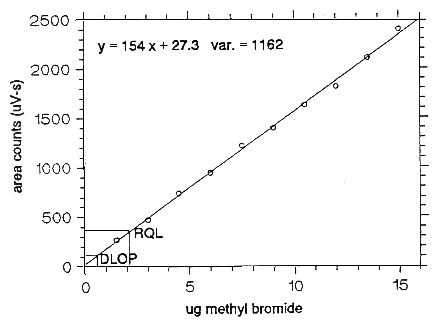
Figure 1.2.1. Plot of data to determine the DLOP/RQL.
1.2.2 Reliable quantitation limit (RQL)
The reliable quantitation limit is 2.21 µg per sample (0.19 ppm). This is the amount of analyte spiked on a sampler that will give a signal that is considered the lower limit for precise quantitative measurements.
The RQL is considered the lower limit for precise quantitative measurements. It is determined from the regression line data obtained for the calculation of the DLOP (Section 1.2.1), providing at least 75% of the analyte is recovered. The RQL is defined as the concentration of analyte that gives a response (YRQL) such that
therefore
| RQL = | 10(SEE)
A |
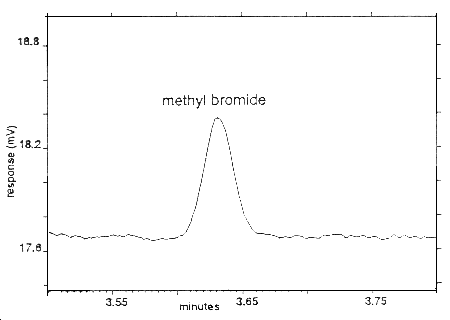
Figure 1.2.2 Chromatogram of the RQL.
| Table 1.2.2 Reliable Quantitation Limit | ||
| mass per sample (µg) |
mass recovered (µg) |
recovery (%) |
|
| ||
| 3 6 9 12 15 18 21 24 27 30 |
2.808 5.508 8.289 11.05 14.28 16.94 19.64 23.18 25.46 28.59 |
93.6 91.8 92.1 92.1 95.2 94.1 93.5 96.6 94.3 95.3 |
|
| ||
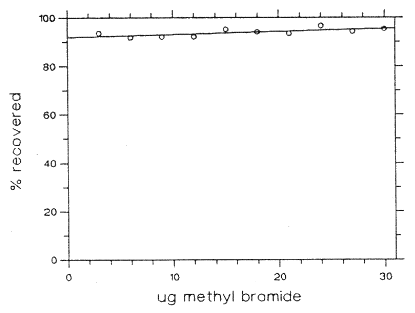
Figure 1.2.3 Plot of data to determine the RQL.
2. Sampling Procedure
- 2.1 Apparatus
- 2.1.1 Samples are collected using a personal sampling pump
calibrated, with the sampling device attached, to within ±5% of the
recommended flow rate.
2.1.2 Samples are collected with 11 cm × 6 mm i.d. × 8 mm o.d.
glass sampling tubes packed with two sections of Anasorb
747TM. The front section contains 400 mg
and the back section contains 200 mg of Anasorb
747TM. The sections are held in place by a
glass wool plug before the front section and a urethane plug after
the back section and are separated by a urethane foam plug. For this
evaluation, commercially prepared sampling tubes were purchased from
SKC, Inc. (Fullerton CA, catalog no.
2.2 Technique
- 2.2.1 Immediately before sampling, break off the ends of the
sampling tube. All tubes should be from the same lot.
2.2.2 Attach the sampling tube to the pump with flexible tubing. It is desirable to utilize sampling tube holders which have a protective cover to shield the employee from the sharp, jagged end of the sampling tube. Position the tube so that sampled air passes through the front section of the tube first.
2.2.3 Air being sampled should not pass through any hose or tubing before entering the sampling tube.
2.2.4 Attach the sampler vertically with the front section pointing downward, in the worker's breathing zone, and positioned so it does not impede work performance or safety.
2.2.5 After sampling for the appropriate time, remove the sample
and seal the tube with plastic end caps. Wrap each sample
2.2.6 Submit at least one blank sample with each set of samples. Handle the blank sampler in the same manner as the other samples except draw no air through it.
2.2.7 Record sample volumes (in liters of air) for each sample, along with any potential interferences.
2.2.8 Ship any bulk samples separate from the air samples.
2.2.9 Submit the samples to the laboratory for analysis as soon as possible after sampling. If delay is unavoidable, store the samples in a refrigerator.
2.3 Desorption efficiency
The desorption efficiencies of methyl bromide were determined by spiking the Anasorb 747TM tubes with the analytes at 0.1 to 2 times the target concentration. The loadings on the tubes were 23.3, 117, 233, and 466 µg of methyl bromide. These samples were stored overnight at ambient temperature and then desorbed and analyzed. The average desorption efficiency over the studied range was 96.0%.
| Table 2.3 Desorption Efficiency of Methyl bromide | |||||
| % Recovered | |||||
| 0.1 × | 0.5 × | 1.0 × | 2.0 × | ||
| Tube # | 23.3µg | 117µg | 233µg | 466µg | |
|
| |||||
| 1 | 96.9 | 96.9 | 96.4 | 95.5 | |
| 2 | 95.8 | 97.8 | 96.9 | 96.6 | |
| 3 | 95.0 | 95.2 | 95.7 | 98.6 | |
| 4 | 95.0 | 94.9 | 96.6 | 95.5 | |
| 5 | 97.2 | 95.1 | 96.0 | 95.4 | |
| 6 | 95.5 | 94.1 | 96.2 | 94.3 | |
| average | 95.9 | 95.7 | 96.3 | 96.0 | |
| overall average | 96.0 | ||||
| standard deviation | ±1.09 | ||||
|
| |||||
2.4 Retention efficiency
The sampling tubes were spiked with 488 µg (41.9 ppm) methyl
bromide, allowed to equilibrate overnight at room temperature. They
were placed in series with another Anasorb
747TM, and then had 3 L humid air (80% RH at
21°C) pulled through them at 0.2 Lpm. They were opened, desorbed, and
analyzed by
| Table 2.4.1 Retention Efficiency of Methyl bromide with 3 L Humid Air | |||||
| % Recovered Front Tube | % Recovered Back Tube | ||||
| Front | Back | Front | Back | Total | |
| Tube# | section | section | section | section | |
|
| |||||
| 1 | 80.3 | 19.7 | 0 | 0 | 100 |
| 2 | 80.8 | 18.6 | 0 | 0 | 99.4 |
| 3 | 84.5 | 15.4 | 0 | 0 | 99.9 |
| 4 | 83.0 | 15.7 | 0 | 0 | 98.7 |
| 5 | 82.6 | 15.8 | 0 | 0 | 98.4 |
| 6 | 85.2 | 13.8 | 0 | 0 | 99.0 |
| average | 99.2 | ||||
|
| |||||
In another retention study the sampling tubes were spiked with 488 µg (41.9 ppm) methyl bromide, allowed to equilibrate overnight at room temperature. They were placed in series with another Anasorb 747TM, and then had 4 L humid air (80% RH at 21°C) pulled through them at 0.1 Lpm. There was methyl bromide found on the front portion of the back tube. This further illustrates the importance of using two tubes in series to sample for methyl bromide.
| Table 2.4.2 Retention Efficiency of Methyl bromide with 4 L Humid Air | |||||
| % Recovered Front Tube | % Recovered Back Tube | ||||
| Front | Back | Front | Back | Total | |
| Tube# | section | section | section | section | |
|
| |||||
| 1 | 58.5 | 33.7 | 6.9 | 0 | 99.1 |
| 2 | 62.6 | 28.8 | 8.2 | 0 | 99.6 |
| 3 | 60.3 | 29.4 | 8.3 | 0 | 98.0 |
| 4 | 58.9 | 32.4 | 8.6 | 0 | 99.9 |
| average | 99.2 | ||||
|
| |||||
2.5 Sample storage
The front sections of twelve sampling tubes were each spiked with 443 µg (38 ppm) of methyl bromide. Twelve more tubes had 3 liters of humid air (80% RH at 21°C) drawn through them before they were spiked with 443 µg (38 ppm) of methyl bromide. Six of each kind were stored at room temperature, approximately 24°C, and the other six of each kind were stored in the refrigerator at 0°C. Three of each type of samples were analyzed after 7 days and the remaining three samples of each type after 14 days. The amounts recovered indicate good storage stability for the time period studied for the refrigerated samples, but poor recoveries for the samples stored at room temperature. The refrigerated samples had an average recovery of 99.5% for dry samples and 94.2% for humid air samples. Samples were corrected for desorption efficiency. There was migration of the methyl bromide with time, with the amounts found on the back section of the tubes increasing from 5% to 11.7% in the refrigerated samples, and from 28% to 44% of amount recovered in the samples stored at room temperature. This indicates the importance of sampling with two tubes in series, as breakthrough can only be determined if the tubes are separated after sampling and analyzed separately. The poor storage at room temperature indicates that samples should be refrigerated as soon as possible after sampling, and samples should be kept cold until analysis.
| Table 2.5 Storage Test for Methyl bromide | ||||
| Time | % Recovered | % Recovered | % Recovered | % Recovered |
| (days) | Humid 24°C | Humid 0°C | Dry 24°C | Dry 0°C |
|
| ||||
| 7 | 77.9 | 95.2 | 84.9 | 99.3 |
| 7 | 71.8 | 96.3 | 86.3 | 99.2 |
| 7 | 79.4 | 94.8 | 89.2 | 100 |
| 14 | 29.3 | 93.5 | 47.0 | 99.9 |
| 14 | 26.6 | 92.4 | 46.0 | 99.6 |
| 14 | 29.2 | 93.0 | 46.1 | 99.2 |
| average | 94.2 | average | 99.5 | |
|
| ||||
2.6 Recommended air volume and sampling rate.
Based on the data collected in this evaluation, a maximum of 3 L should be collected at a maximum sampling rate of 0.2 L/min. Based on the detection limit, the minimum air volume is 1 liter, and the minimum sampling rate is 0.05 Lpm.
2.7 Interferences (sampling)
- 2.7.1 It is not known if any compounds will severely interfere
with the collection of methyl bromide on the sampling tubes. In
general, the presence of other contaminant vapors in the air will
reduce the capacity of the Anasorb 747TM
tube to collect methyl bromide.
2.7.2 Suspected interferences should be reported to the laboratory with submitted samples.
2.8 Safety precautions (sampling)
- 2.8.1 Attach the sampling equipment to the worker in such a
manner that it will not interfere with work performance or safety.
2.8.2 Follow all safety practices that apply to the work area being sampled.
2.8.3 Wear eye protection when breaking the ends of the glass sampling tubes.
3. Analytical Procedure
- 3.1 Apparatus
- 3.1.1 The instrument used in this study was a gas chromatograph
equipped with a flame ionization detector, specifically a Hewlett
packard model 5890.
3.1.2 A GC column capable of separating the analyte from any
interferences. The column used in this study was a 60 meter
capillary column with 0.32 mm i.d. coated with
3.1.3 An electronic integrator or some suitable method of measuring peak areas.
3.1.4 Four milliliter vials with TeflonTM-lined caps for sample desorption.
3.1.5 Two milliliter vials with TeflonTM-lined caps, which fit into the autosampler for sample analysis.
3.1.6 A 10 µL syringe or other convenient size for sample injection.
3.1.7 Pipets for dispensing the desorbing solution. A Repipet® was used in this study.
3.1.8 Volumetric flasks - 5 or 10 mL and other convenient sizes for preparing standards.
3.1.9 Open-sided vial rack which holds the 4 mL vials
3.1.10 Tray with 2" sides, which the open-sided vial rack can fit into
3.1.11 A 1000 µL gas-tight syringe or other convient 3.4size for preparation of analytical standards.
3.2 Reagents
- 3.2.1 GC grade nitrogen, hydrogen, and air.
3.2.2 Methyl bromide
3.2.3 Carbon disulfide, Reagent grade
3.2.4 p-Cymene, Reagent grade
3.2.5 Desorbing solution was carbon disulfide with 0.25 µL/mL p-cymene internal standard.
3.3 Standard preparation
- 3.3.1 At least two separate stock standards are prepared by
diluting a known quantity of methyl bromide with the desorbing
solution of carbon disulfide with 0.25 µL/mL
| µL methylbromide
mL desorbing solution |
× | pressure
760mm |
× | 298°K
273°K+temp.°C |
× | µmole
24.45 |
× | 94.95
µmole |
where:
| pressure | = the pressure at the time the standard are prepared |
| temp. °C | = the temperature of the room at the time the standards are prepared |
| 94.95 | = the molecular weight of methyl bromide |
For example, to calculate the µg/mL of methyl bromide from a standard of 500 µL methyl bromide in 4 mL desorbing solution at 654 mm and 22°C:
| 422 µg/mL = | 500 µL methylbromide
4 mL desorbing solution |
× | 654mm
760mm |
× | 298°K
273°K+22°C |
× | µmole
24.45 |
× | 94.95
µmole |
3.3.2 A third standard at a lower concentration was prepared to check the linearity of the calibration at a concentration of 100 µL methyl bromide in 4 mL desorbing solution or 25 µL/mL or 104 µg/mL. A fourth standard was prepared by diluting 1/10 the first standard for a concentration of 42.2 µg/mL
3.4 Sample preparation
- 3.4.1 Vials, 4 mL size, are placed into an open-sided vial rack.
This rack is placed into a tray and water is added to the tray, so
that the water level is
3.4.2 Sample tubes are opened and the front and back section of each tube are placed in separate 4 mL vials. It is important that the vial be surrounded with ice when the carbon disulfide is added. The ice prevents the heat of desorption from vaporizing the methyl bromide out of solution causing it to be lost.
3.4.3 Each section is desorbed with 2 mL of the desorbing solution of carbon disulfide with 0.25 µL/mL p-cymene.
3.4.4 The vials are sealed immediately and allowed to desorb for 30 minutes with constant shaking.
3.4.5 The samples are tranfered to 2 mL vials, which fit into the autosampler, and analyzed.
3.5 Analysis
- 3.5.1 Gas chromatograph conditions.
| Injection size: | 1 µL |
| Flow rates (mL/min) | |
| Nitrogen (make-up): | 30 |
| Hydrogen(carrier): | 2 |
| Hydrogen(detector): | 60 |
| Air: | 450 |
| Temperatures (°C) | |
| Injector: | 180 |
| Detector: | 220 |
| Column: | 50° - 4 min - 10°/min - 160° |
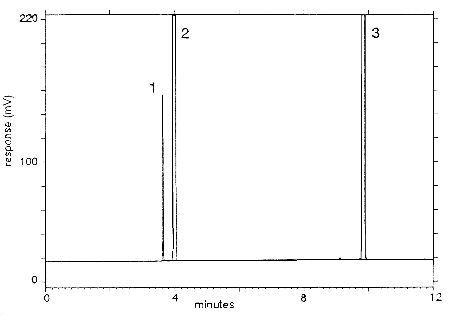
Figure 3.5.1 A chromatogram of an analytical standard at the PEL.
The peaks are identified as follows:
1 = methyl bromide, 2 = carbon disulfide, and3 = p-cymene.
3.5.2 Peak areas are measured by an integrator or other suitable means.
3.6 Interferences (analytical)
- 3.6.1 Any compound that produces a response and has a similar
retention time as the analyte is a potential interference. If any
potential interferences were reported, they should be considered
before samples are desorbed. The compounds that may occur in the
workplace with methyl bromide which do not interfere using the
analytical conditions listed in this study were ethylene dichloride,
ethylene dibromide, methylene chloride, and trichloroethylene.
Generally, chromatographic conditions can be altered to separate an
interference from the analyte.
3.6.2 When necessary, the identity or purity of an analyte peak
may be confirmed by
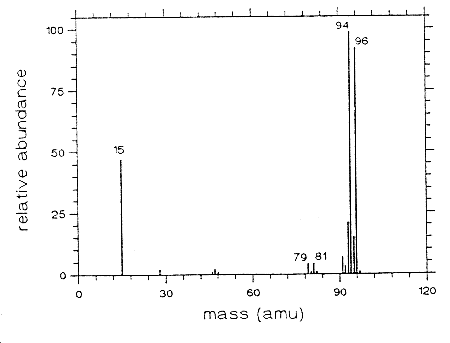
Figure 3.6.2 Mass spectra of methyl bromide.
3.7 Calculations
- 3.7.1 The instrument was calibrated with a standard of 422
µg/mL (36.2 ppm) methyl bromide in the desorbing solution.
The linearity of the calibration was checked with a standards of
42.2 and 104 µg/mL.
3.7.2 If the calibration is non-linear, two or more standard at different concentrations must be analyzed, bracketing the samples, so a calibration curve can be plotted and sample values obtained.
3.7.3 Values (µg) obtained from the blanks are subtracted from air samples.
3.7.4 To calculate the concentration of analyte in the air sample the following formulas are used:
| (µg/m) (desorption volume)
(desorption efficiency) |
= mass of analyte in sample |
| (mass of analyte in sample)
molecular weight |
= number of moles of analyte |
| (number of moles of analyte) |
(molar volume at 25°C & 760mm) |
= | volume the analyte
will occupy at 25°C & 760mm |
| (volume analyte occupies)
(106)*
(air volume) |
= ppm |
* All units must cancel.
3.7.5 The above equations can be consolidated to the following formula.
| (µg/mL)(DV)(24.45)(106)(g)(mg)
(3 L)(DE)(MW)(1000mg)(1000µg) |
= ppm |
| µg/mL 24.45 MW DV 3 L DE |
= concentration of analyte in sample or standard = Molar volume (liters/mole) at 25°C and 760 mm = Molecular weight (g/mole) = Desorption volume = 3 liter air sample = Desorption efficiency |
3.7.6 This calculation is done for each section of the sampling tube and the results added together.
3.8 Safety precautions (analytical)
- 3.8.1 Avoid skin contact and inhalation of all chemicals.
3.8.2 Wear safety glasses, gloves and a lab coat at all times while in the laboratory areas.
4. Recommendations for Further Study
Collection studies should be performed from a dynamically generated test atmosphere. Other sampling tubes should be explored in an attempt to find a sampling media that methyl bromide will have good storage stability at room temperatures.
5. References
- 5.1 "NIOSH Manual of Analytical Methods", U.S. Department of
Health, Education, and Welfare, Public Health Service, Center for
Disease Control, National Institute for Occupational Safety and
Health, Third Edition, Vol. 1, Method 2520.
5.2 Tharr, D., Applied Occup. Environ. Hyg., 9 (3), March 1994, p. 165
5.3 "Documentation of the Threshold Limit Values and Biological Exposure Indices", Fifth Edition, American Conference of Governmental Industrial Hygienists Inc., Cincinnati, OH, 1986, p. 376.
5.4 Windholz, M., "The Merck Index", Eleventh Edition, Merck & Co., Rahway N. J., 1989, p. 950.