HMX
| Method number: | PV2032 |
| Matrix: | Air |
| Target concentration: | 0.2 mg/m3 (arbitrary). There is no OSHA PEL or ACGIH TLV for HMX. |
| Procedure: | Samples are collected by drawing air through glass fiber filters. Samples are extracted with acetone and analyzed by high performance liquid chromatography (HPLC) with a UV detector. |
| Recommended air volume and sampling rate: |
500 L at 1 Lpm |
| Detection limit of the analytical procedure: |
10.5 ng/injection |
| Status of method: | Stopgap method. This method has been only partially evaluated and is presented for information and trial use. |
| Date: July 1987 (final) | Chemist: Yihlin Chan |
Carcinogen and Pesticide Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1.History of procedure
The OSHA Analytical Laboratory received a set of samples with a request for the analysis of HMX. The samples had been collected on glass fiber filters. This report describes the analytical procedure developed.
1.1.2.Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
The oral LD50 of HMX is 1500 mg/kg in mice and 300 mg/kg in guinea pigs. The intravenous LD50 is 28 mg/kg in guinea pigs. (Ref. 5.1.)
1.1.3.Potential workplace exposure
HMX is a high energy explosive. It is "much less toxic than TNT and may be handled with no physiological effect if appropriate precautions are taken to assure cleanliness of operations." (Ref. 5.2.) No data on the extent of workplace exposure could be found.
1.1.4. Physical properties (Ref. 5.1., 5.2.)
| Chemical name: | Octahydro-1,3,5,7-tetranitro-1,3,5,7 tetrazocine |
| CAS #: | 2691-41-0 |
| Synonym: | Cyclotetramethylenetetranitramine; HMX; HW 4; LX 14-0; Octogen. |
| Mol. weight: | 296.20 |
| Mol. formula: | C4H8N8O8 |
| Structure: | 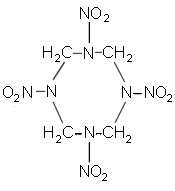 |
| Appearance: | White crystalline solid. |
| M.P.: | 286°C |
| Solubility: | Soluble in acetone. |
1.2. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 10.5 ng per injection.
2. Sampling Procedure
- 2.1. Apparatus and reagents
- 2.1.1. A personal sampling pump that can be calibrated to within
5% of the recommended flow rate.
2.1.2. Glass fiber filter, 37-mm diameter, Gelman Type A or equivalent.
2.1.3. Filter holder for 37-mm filters, Millipore M000037A0 or equivalent.
2.2. Sampling technique
Use standard air sampling procedure as specified in the OSHA Instruction CPL 2-2.20A, Chapter II: Standard Method for Sampling Air Contaminants.
2.3. Recommended air volume and sampling rate
- 2.3.1. The recommended air volume is 500 L.
2.3.2. The recommended sampling rate is 1 Lpm.
2.4. Extraction efficiency
Six glass fiber filters were each spiked with 23.44 ug of HMX. After overnight storage, two filters were extracted with 5 mL of acetone. The average recovery of HMX was 95.6%.
| Sample # | HMX recovered | Recovery |
| ---------- | ----------------- | ---------- |
| YC1 | 22.8 ug | 97.3% |
| YC2 | 22.0 ug | 93.9% |
| ---------- | ----------------- | ---------- |
| Average: | 95.6% |
Humid air (90% RH, 500 L) was drawn through the remaining four filters in Section 2.4. Two filters were analyzed. The average recovery of HMX was 93.7%.
| Sample # | HMX recovered | Recovery |
| ---------- | ----------------- | ---------- |
| YC3 | 21.4 ug | 96.0% |
| YC4 | 20.5 ug | 91.3% |
| ---------- | ----------------- | ---------- |
| Average: | 93.7% |
2.6. Storage test (7 days)
Three glass fiber filters were each spiked with 23.44 ug of HMX. Humid air (RH 74%, 276 L at 1 Lpm) was pulled through the filters. The filters were stored at room temperature for 7 days, extracted, and analyzed. The average recovery of the HMX was 102.7%.
| Sample # | HMX recovered | Recovery |
| ---------- | ----------------- | ---------- |
| YCH1 | 24.21 ug | 103.3% |
| YCH2 | 24.15 ug | 103.0% |
| YCH3 | 23.86 ug | 101.8% |
| ---------- | ----------------- | ---------- |
| Average: | 102.7% |
2.7. Interferences (Sampling)
There are no known interferences to the sampling procedure.
3. Analytical procedure
- 3.1. Apparatus
- 3.1.1. High performance liquid chromatograph
3.1.2. Zorbax ODS HPLC column
3.1.3. UV detector. Waters 490 Programmable Multiwavelength Detector was used in this study.
3.1.4. Stripchart recorder
3.2. Reagents
- 3.2.1. HMX standard
3.2.2. Acetonitrile, HPLC grade
3.2.3. Water, HPLC grade
3.2.4. Acetone, reagent grade
3.3. Standard preparation
Weigh 3 to 5 mg of HMX in a 10-mL volumetric flask. Add acetone to the mark. Dilute to a working range of 0.7 to 20 ug/mL.
3.4. Sample preparation
Place glass fiber filter in a scintillation vial. Add 5 mL of acetone. Shake on a mechanical shaker for 30 minutes.
3.5. Analysis
- 3.5.1. Instrument conditions
| Column: | Zorbax ODS column |
| Eluent: | 40% acetonitrile, 60% water |
| Flow rate: | 1.0 mL/min |
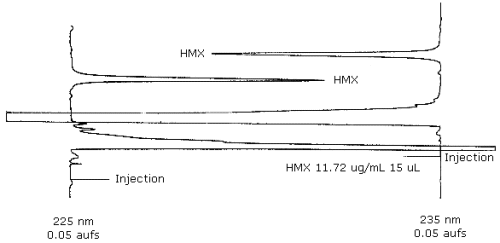
| Detector: | 235 nm (primary), 225 nm |
| Retention time: | 11.1 min |
3.5.2. Chromatogram (see Figure 1)
3.6. Interferences
- 3.6.1. Any compound that has the same retention time as the HMX
and absorbs at 235 nm and 225 nm is an interference.
3.6.2. Most interferences can be circumvented by altering the chromatographic conditions.
3.6.3. Retention time alone is not proof of a chemical identity. Confirmation by other means should be sought when possible.
3.7. Calculations
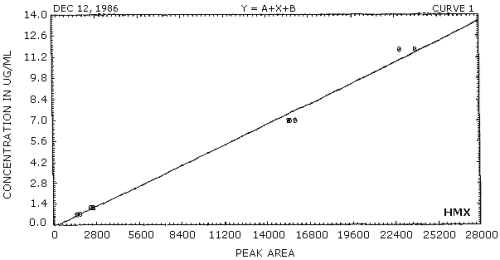
- 3.7.1. A calibration curve is constructed by plotting
concentration versus detector response.
3.7.2. The concentration of a sample is determined from the calibration curve.
3.7.3. The concentration of HMX is given by:
| mg/m3 = | (µg/mL) × (5 mL)
(air volume, L) × (extraction efficiency) |
4. Recommendations for further study
The method should be fully validated.
5. References
- 5.1. Registry of Toxic Effects of Chemical Substances
1983-84, DHHS (NIOSH) Publication No.
5.2. Grayson, Martin, ed., Kirk-Othmer Encyclopedia of Chemical Technology, Volume 9, New York: John Wiley & Sons, 1980.