PROPOXUR (BAYGON)
| Method number: | PV2007 |
| Matrix: | Air |
| Target Concentration: | 0.5 mg/m3 ACGIH TLV. There is no OSHA PEL for propoxur. |
| Procedure: | Samples are collected by drawing known volumes of air through
OSHA versatile sampler |
| Recommended air volume and sampling rate: | 60 L and 1.0 L/min |
| Detection limit of the overall procedure (based on the recommended air volume): | 0.003 mg/m3 |
| Stopgap method. This method has been only partially evaluated and is presented for information and trial use. | |
| Date: July, 1987 (final) | Chemist: David B. Armitage |
Carcinogen And Pesticide Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History of procedure
This evaluation was undertaken to determine the effectiveness of
the
It should be noted that in this evaluation for propoxur several other analytes were also present in the analytical procedure. These other analytes are not mentioned in this evaluation, but can be seen on the sample chromatogram. (Sec. 3.5.2.)
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy).
The acute oral LD50 for male rats is 83 mg/kg and the acute oral LD50 for female rats is 86 mg/kg. The acute dermal LD50 for both sexes of rats is >2400 mg/kg. (Ref. 5.2.)
As with other carbamate pesticides, propoxur inhibits cholinesterase and this effect is apparently the basis of its toxic action. Carbamates are absorbed by inhalation, ingestion, and dermal penetration. (Ref. 5.2.)
In a volunteer study, a single dose of 1.5 mg/kg produced mild symptoms, primarily gastrointestinal in nature, which disappeared within two hours of ingestion. The erythrocyte cholinesterase level reached a minimum of 27% of normal. (Ref. 5.2.)
On the basis of the available toxicity data, propoxur has been given a TLV of 0.5 mg/m3 by the ACGIH. (Ref. 5.2.)
1.1.3. Potential workplace exposure
No estimate of workplace exposure was found. Propoxur is a widely used pesticide especially effective against insects affecting man and animals (i.e. cockroaches, flies, and mosquitoes). (Ref. 5.2.)
1.1.4. Physical properties (Ref. 5.2.-5.5.)
| Molecular weight: | 209.24 |
| Molecular formula: | C11H15N03 |
| CAS #: | 114-21-1 |
| Melting point: | 91.5°C |
| Appearance: | white, crystalline, odorless solid |
| Solubility: | soluble in organic polar compounds soluble to 0.2% in water at 20°C |
| Synonyms: | aprocarb, Bay 39007, Baygon, Propagon, Propygon, Suncide, Tygon Fliegenkugel, Unden |
| Chemical name: | 2-1(1-methylethoxy)phenol methyl carbamate |
| Structure: | 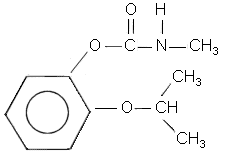 |
1.2. Limit defining parameters
The detection limit of the analytical procedure is 1 ng per injection. This is the amount of analyte which will give a peak whose height is approximately five times the baseline noise.
2. Sampling procedure
2.1. Apparatus
2.1.1. Samples are collected by using a personal sampling pump that can be calibrated to within ± 5% of the recommended flow rate with the sampling device in line.
2.1.2. Samples are collected with
2.2. Reagents
No sampling reagents are required.
2.3. Sampling technique
2.3.1. Attach the small end of the
2.3.2. After sampling for the appropriate time, remove the sampling device and seal the tube with plastic end caps.
2.3.3. Wrap each sample
2.3.4. With each set of samples, submit at least one blank. The blank should be handled the same as the other samples except that no air is drawn through it.
2.3.5. Bulk samples should be submitted for analysis in a separate container. Do not ship with the air samples.
2.4. Extraction efficiency
Two 13 mm glass fiber filters were each liquid spiked with 29.76 µg
of propoxur. The two filters, along with a blank filter, were each
placed in separate
The average extraction efficiency for these two filters (with the
2.5. Retention efficiency
Two
The average retention efficiency for these two filters was 99%.
2.6. Sample storage
Two
The average recovery after ten days of storage was 90%.
2.7. Recommended air volume and sampling rate
2.7.1. The recommended air volume is 60 L.
2.7.2. The recommended flow rate is 1.0 L/min.
2.8. Interferences
It is not known if any compounds will interfere with the collection of propoxur. Suspected interferences should be reported to the laboratory with submitted samples.
2.9. Safety precautions
2.9.1. Attach the sampling equipment in such a manner that it will not interfere with work performance or employee safety.
2.9.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical procedure
3.1. Apparatus
3.1.1. A
3.1.2. An HPLC column capable of separating propoxur from any interferences. A 25 cm × 4.6 mm i.d. DuPont Zorbax ODS (6 micron) column was used in this evaluation.
3.1.3. An electronic integrator or other suitable means of
measuring detector response. A
3.1.4. Vials,
3.1.5. Volumetric flasks, pipets, and syringes for preparing standards, making dilutions, and performing injections.
3.2. Reagents
3.2.1. HPLC grade acetonitrile (ACN).
3.2.2. HPLC grade water. A Millipore
3.2.3. Propoxur, 99+% pure (EPA).
3.3. Standard preparation
Stock standard solutions are prepared by adding acetonitrile to preweighed amounts of propoxur. Working range standard solutions are prepared by diluting stock solutions with acetonitrile. Stock and dilute standards are stored in a freezer.
3.4. Sample preparation
3.4.1. Transfer the
3.4.2. Add 2.0 mL of acetonitrile to each vial.
3.4.3. Seal the vials with
3.5. Analysis
3.5.1. Instrument conditions
| Column: | 25 cm × 4.6 mm i.d. stainless steel column, packed with 6 micron DuPont Zorbax ODS |
| Mobile Phase: | 45% ACN / 55% water (v/v) |
| Flow rate: | 1 mL/min |
| UV detector: | 214 nm |
| Retention time: | 5.9 min |
| Injection volume: | 10 µL |
3.5.2. Chromatogram (See Figure 2)
3.6. Interferences
3.6.1. Any compound having a similar retention time to the analyte is a potential interference. Generally, chromatographic conditions can be altered to separate an interference from the analyte.
3.6.2. Retention time on a single column is not proof of chemical identity. Analysis by an alternate HPLC column, detection at another wavelength, comparison of absorbance response ratios, and confirmation by mass spectrometry are additional means of identification.
3.7. Calculations
3.7.1. A calibration curve is constructed by plotting detector response versus standard concentration.
3.7.2. The concentration of propoxur in a sample is determined from the calibration curve. If propoxur is found on the backup section, it is added to the amount found on the front section. Blank corrections for each section should be performed before adding the results together.
3.7.3. The air concentration is then determined by the following formula.
| mg/m3 = | (µg/mL in sample) × (extraction
volume, mL)
(air volume, L) × (extraction efficiency, decimal) |
3.8. Safety precautions
3.8.1. Avoid exposure to all standards.
3.8.2. Avoid exposure to all solvents.
3.8.3. Wear safety glasses at all times.
4. Recommendations for further study
As propoxur is a common pesticide, this method should be fully validated.
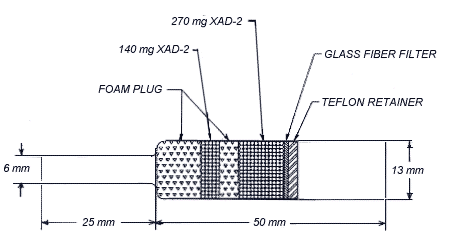
Figure 1. 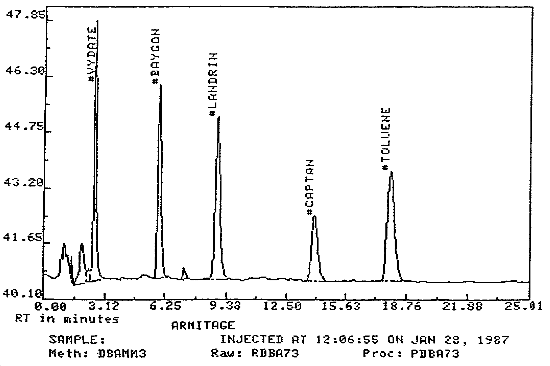
Figure 2. Chromatogram of Propoxur (with other analytes)
5. References
5.1. Burright, D., Method #63, "Carbaryl (Sevin)", OSHA Analytical Laboratory, unpublished, 1987.
5.2. "Documentation of the Threshold Limit Values and Biological Exposure Indices", American Conference of Governmental Industrial Hygienists Inc., fifth edition, 1986.
5.3. Windholz, M., Ed. "Merck Index", 10th ed.; Merck and Co., Rahway, NJ, 1983.
5.4. "Farm Chemicals Handbook", Meister Publishing Co., 1985.
5.5. "Chemical Information File", U.S. Department of Labor, Occupational Safety and Health Administration, Directorate of Technical Support, June 14, 1985.