| Method number: |
1004 |
| |
| MEK: |
|
Target
concentration:
OSHA PEL:
ACGIH TLV: |
200 ppm (590 mg/m3)
TWA
200 ppm (590 mg/m3) TWA
200 ppm
TWA
300 ppm STEL/C |
| |
| MIBK: |
|
Target
concentration:
OSHA PEL:
ACGIH TLV: |
100 ppm (410 mg/m3)
TWA
100 ppm (410 mg/m3) TWA
50 ppm TWA
75
ppm STEL/C |
| |
| Procedure: |
Active samples are collected by
drawing workplace air through SKC Anasorb CMS (carbon
molecular sieves) sampling tubes with personal sampling pumps.
Diffusive samples are collected by exposing either SKC 575-002
Passive Samplers or 3M 3520 OVMs to workplace air. Samples are
extracted with carbon disulfide containing 1% N,N-dimethylformamide and analyzed by GC
using a flame ionization detector. |
| |
Recommended sampling time and
sampling rate:
|
|
SKC CMS sampling tube:
SKC
575-002 Passive Sampler:
3M 3520 OVM: |
240 min at 50 mL/min (12 L)
240
min
240 min |
| |
| Reliable quantitation limit (RQL)
and standard error of estimate (SEE): |
|
| |
MEK
|
|
MIBK
|
| |
RQL |
SEE |
|
RQL |
SEE |
| |
(ppb) |
(µg/m3) |
(%) |
|
(ppb) |
(µg/m3) |
(%) |
|
SKC CMS sampling tubes
SKC 575-002
Passive Sampler
3M 3520 OVM |
23
109
33 |
68
320
98 |
6.0
9.1*
8.3* |
|
9
94
35 |
36
384
144 |
5.9
9.1*
8.0* |
| |
| |
| |
*For samples where sampling site
atmospheric pressure and temperature are known. When either or
both of these values are unknown, see Section
4.4 for applicable standard errors of estimate. |
| |
| Special requirements: |
Report sampling site pressure and
temperature when using diffusive samplers. Refrigerate samples
for MEK and MIBK upon receipt at laboratory. |
| |
| Status of method: |
Evaluated method. This method has
been subjected to the established evaluation procedures of the
Methods Development Team. |
| |
| September 2000 |
Warren
Hendricks |
Methods
Development Team
Industrial Hygiene Chemistry
Division
OSHA Salt Lake Technical Center
Sandy, UT
84070-6406 |
1.
General Discussion
1.1 Background
1.1.1 History
Workplace determination of 2-butanone (methyl
ethyl ketone, MEK) and of hexone (methyl isobutyl ketone,
MIBK) is of considerable interest to OSHA. Both MEK and MIBK
rank in the top 15 most requested organic solvent analytes for
samples received at SLTC. This work was performed to provide
OSHA with convenient active and diffusive sampling methods to
monitor workplace air for these chemical hazards. The two
chemicals were evaluated simultaneously to conserve SLTC
resources. Evaluation of MEK revealed certain sampling and
analytical problems, such as storage instability and sample
extraction difficulties, that are addressed in this method.
Evaluation of MIBK was straightforward and uneventful.
Current active
sampling methods for MEK specify use of one of the following
three sampling media and techniques: 1) two silica gel
sampling tubes connected in-series (both tubes 150/75 mg
sections, 3-L air sample); 2) Carboseive S-III sampling tubes
(130/65 mg sections, 3-L air sample); or 3) Anasorb 747
sampling tubes (140/70 mg sections, 12-L air sample).1
The present sampling method for MIBK requires the use of
charcoal tubes (100/50 mg sections, 25-L air sample).2
The active sampling medium evaluated in this work (SKC
Anasorb CMS) permits collection of a four-hour (12-L) sample
for both MEK and MIBK on the same sampling tube. Anasorb CMS
is a proprietary carbon molecular sieve that may be similar to
Carbosieve S-III carbon molecular sieve marketed by Supelco.
Diffusive sampling methods allow a 4-hour sample to be
collected on either SKC 575-002 Passive Samplers or on 3M 3520
Organic Vapor Monitors (OVMs). Storage stability tests showed
that MEK is more stable on 3M 3520 OVMs than on SKC 575-002
Passive Samplers. The method requires refrigerated storage of
samples upon laboratory receipt. Refrigerated storage is
precautionary for SKC CMS sampling tubes and for 3M 3520 OVMs,
but is obligatory for SKC 575-002 Passive Samplers.
Refrigerated sample shipment for SKC 575-002 Passive Samplers
is unnecessary, unless sample shipment is anticipated to be
delayed for more than three days.
Anasorb 747 sampling
tubes were tested, but were found to be unsatisfactory for use
in this method. Small Anasorb 747 (140/70 mg sections)
sampling tubes did not have sufficient capacity to permit a
four-hour MEK sample in the presence of MIBK. Large Anasorb
747 (400/200 mg sections) sampling tubes have sufficient
capacity, but ambient MEK storage stability was poor. Anasorb
747 is the sorbent contained in SKC 575-002 Passive Samplers,
and MEK storage stability data for active and for diffusive
samplers employing this sorbent were comparable. Tests showed
that Anasorb 747 does have adequate sampling capacity for MIBK
in the presence of MEK, and that ambient storage stability was
satisfactory. Some of the Anasorb 747 sampling tube evaluation
data is included in this report for the preceding reasons, but
it is for information only, and the use Anasorb 747 sampling
tubes is not recommended for this application. 1.1.2 Toxic effects 3
(This section is for information only and should not be taken as
the basis of OSHA policy.)
MEK
ACGIH's Documentation of the TLVs
reports mild eye, nose, and throat irritation from exposure to
MEK at 100 to 200 ppm. Low-grade intoxication has occurred at
300 to 600 ppm. The 100% response odor threshold was 10 ppm.
Central nervous system effects were noted following exposures
to mixtures of organic chemicals including MEK. There was no
excess cancer risk reported.
MIBK
ACGIH
reports that MIBK is an irritant to the eyes, nose, throat,
and skin. An odor threshold of 0.3 to 0.7 ppm has been
reported. Headache and nausea are common complaints of MIBK
exposure. Exposure to high concentrations could result in
death because of its narcotic effects. Liver and kidney
effects have been reported.
ACGIH, in the 1996 supplement to the
Documentation of the TLVs 4,
stated that dermal and gastrointestinal absorption of MIBK
could be significant. The BEI Committee recommended monitoring
of MIBK in urine at the end of the work shift as an indicator
of recent exposure to MIBK. The recommended BEI value is 2
mg/L MIBK in urine. Adjustment for creatinine is
inappropriate. 1.1.3 Workplace
exposure 5
MEK
MEK is used as a solvent in the
surface coating industry and in dewaxing of lubricating oils.
It is used in the manufacture of colorless synthetic resins,
artificial leather, rubbers, varnishes and glues. It is
commonly used with other solvents such as acetone, ethyl
acetate, hexane, toluene, and alcohols.
MIBK
MIBK is used as a solvent in synthetic resinous
paints, lacquers, aircraft dopes, and varnishes. It is also a
solvent for adhesives and rubber cement. It is used as a
denaturant for ethyl alcohol, and to extract pharmaceuticals
and uranium fission products. 1.1.4 Physical properties
and descriptive information 6,7
MEK
| CAS number: |
78-93-3 |
vapor pressure: |
10.3 kPa at 20°C |
| IMIS number: |
0430 |
flash point: |
-4°C (closed cup) |
| molecular weight: |
72.10 |
odor: |
acetone-like |
| boiling point: |
79.6°C |
lower explosive limit: |
1.8% (by volume) |
| melting point: |
-86°C |
synonyms: |
butan-2-one; MEK |
| appearance: |
colorless liquid |
solubility: |
water, all common
industrial organic solvents |
| specific gravity: |
0.805 at 20°C |
| molecular formula: |
C4H8O |
MIBK
| CAS number: |
108-10-1 |
vapor pressure: |
2.0 kPa at 25°C |
| IMIS number: |
1385 |
flash point: |
18°C (closed cup) |
| molecular weight: |
100.16 |
odor: |
pleasant, sweet |
| boiling point: |
115.8°C |
lower explosive limit: |
1.4% (by volume) |
| freezing point: |
-84.7°C |
synonyms: |
4-methyl-2-pentanone; MIBK |
| appearance: |
colorless liquid |
| specific gravity: |
0.8017 at 20°C |
solubility: |
1.91 g/mL in
water,miscible with many organic solvents |
| molecular formula: |
C6H12O |
| structural formulas: |

MEK |
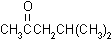
MIBK |
This method was evaluated according to the OSHA
SLTC "Evaluation Guidelines for Air Sampling Methods Utilizing
Chromatographic Analysis"8.
The Guidelines define analytical parameters, specify required
laboratory tests, statistical calculations and acceptance criteria.
The analyte air concentrations throughout this method are based on
the recommended sampling and analytical parameters. Air
concentrations listed in ppm are referenced to 25°C and 101.3 kPa
(760 mmHg).
1.2 Limit defining parameters
1.2.1 Detection limit of the analytical procedure
The detection limits of the analytical procedure
are 4.90 pg for MEK and 3.13 pg for MIBK. These are the
amounts of analyte that will give a detector response that is
significantly different from the response of a reagent blank.
(Section
4.1) 1.2.2 Detection limit of the overall
procedure
The detection limits of the overall procedure are
shown in Table 1.2.2. These are the amounts of analytes spiked
on the respective sampler that will give detector responses
that are significantly different from the responses of
respective sampler blanks. (Section
4.2)
Table
1.2.2
Detection Limits of the Overall Procedure |
|
| |
MEK
|
|
MIBK
|
| sampler |
ng |
ppb |
µg/m3 |
|
ng |
ppb |
µg/m3 |
|
SKC CMS
SKC 575-002
3M 3520 |
244
388
230 |
7
33
10 |
20
96
29 |
|
129
376
281 |
3
28
11 |
11
115
43 |
|
1.2.3 Reliable quantitation limit
The
reliable quantitation limits are shown in Table 1.2.3. These are
the amounts of analytes spiked on the respective samplers that
will give detector responses that are considered the lower
limits for precise quantitative measurements. (Section
4.2)
Table
1.2.3
Reliable Quantitation Limits |
|
| |
MEK
|
|
MIBK
|
| sampler |
ng |
ppb |
µg/m3 |
EE |
|
ng |
ppb |
µg/m3 |
EE |
|
SKC CMS
SKC 575-002
3M 3520 |
813
1295
766 |
23
109
33 |
68
320
98 |
98.5
90.4
105.3 |
|
430
1255
937 |
9
94
35 |
36
384
144 |
111.7
81.0
86.4 |
EE is extraction
efficiency |
1.2.4 Instrument calibration
The standard
errors of estimate are 64 µg/sample for MEK and 47 µg/sample for
MIBK over the range of 1900 to 14250 µg/sample for MEK and 1300
to 9753 µg/sample for MIBK. This range corresponds to 0.25 to 2
times the target concentration for SKC CMS sampling tubes. This
is the sampler with the highest mass loading. (Section
4.3)
1.2.5 Precision (Section
4.4)
SKC CMS sampling tubes
The precisions
of the overall procedure at the 95% confidence level for the
ambient temperature 15-day storage test (at the target
concentration) for SKC CMS tubes are ±11.7% for MEK, and ±11.5%
for MIBK. These precisions each include an additional 5% for
sampling pump variability.
Diffusive samplers
The precisions of the overall
procedure at the 95% confidence level for the ambient
temperature 15-day storage tests (at the target concentration)
are shown in Table 1.2.5. There are different values given,
depending on whether both, either, or neither temperature (T) or atmospheric pressure (P) at the sampling site are known. If the
sampling site temperature is unknown, it is assumed to be 22.2 ±
15°C (72 ± 27°F) and a variability of ±7.7% is included. If the
atmospheric pressure is not known, it is estimated from the
sampling site elevation and a variability of ±3% is included.
Each 3M precision value includes an additional 7.4% for sampling
rate variation, and each SKC value an additional 8.7% also for
sampling rate variation9.
Table
1.2.5
Precision of the Overall Procedure for Diffusive
Samplers |
|
| |
SKC 575-002
Passive Sampler
|
3M 3520 OVM
|
| known condition |
MEK
precision(±%) |
MIBK
precision(±%) |
MEK
precision(±%) |
MIBK
precision(±%) |
|
both T&P
only T
only P
neither T nor
P |
17.9
18.8
23.4
24.1 |
17.7
18.7
23.3
24.0 |
16.3
17.3
22.2
23.0 |
15.7
16.8
21.8
22.6 |
|
1.2.6 Recovery
The
recovery of MEK and of MIBK from samples used in 15-day storage
tests remained above those shown in Table 1.2.6. (Section
4.5)
Table
1.2.6
Recovery (%) |
|
| sampler |
storage temp |
MEK |
MIBK |
|
SKC anasorb CMS Tubes
SKC
575-002
3M 3520 |
ambient
refrigerated
ambient |
84.9
91.3
96.4 |
93.2
88.1
102.6 |
|
1.2.7
Reproducibility
Six samples for each of the three
samplers evaluated in this method were collected from a
controlled test atmosphere and were submitted to OSHA SLTC for
analysis. The samples were analyzed utilizing a draft copy of
this procedure for instruction. They were analyzed following 2
days of storage at 4°C. No individual sample result deviated
from its theoretical value by more than the precision reported
in Section
1.2.5. (Section
4.6) 2.
Sampling Procedure
All safety practices that apply to the
work area being sampled should be followed. The sampling equipment
should be attached to the worker in such a manner that it will not
interfere with work performance or safety.
2.1 Apparatus
2.1.1 SKC Anasorb CMS sampling tubes
Samples
are collected with 7-cm × 4-mm i.d. × 6-mm o.d. glass sampling
tubes packed with two sections of SKC Anasorb CMS. The front
section contains 150 mg and the back section contains 75 mg of
CMS. The sections are held in place with glass wool plugs. For
this evaluation, commercially prepared Anasorb CMS sampling
tubes were purchased from SKC Inc. (catalog no. 226-121).
Samples are collected using a personal sampling pump
calibrated, with the sampling device attached, to within ±5% of
the recommended flow rate.
2.1.2 SKC 575-002 Passive
Samplers and 3M 3520 OVMs
Samples are collected with
either SKC 575-002 Passive Samplers, or with 3M 3520 OVMs.
Samplers were purchased from SKC, Inc. (catalog no. 575-002,
contains one 500-mg section of Anasorb 747), or from 3M (catalog
no. 3520, contains two-sections with charcoal adsorbent pads).
A thermometer and a barometer are used to determine the
sampling site air temperature and atmospheric pressure.
2.2 Reagents
None required.
2.3
Technique
2.3.1 SKC Anasorb CMS sampling tubes
Break
off the ends of the flame-sealed sampling tube, to provide an
opening approximately half the internal diameter of the tube,
immediately before sampling. Wear eye protection when breaking
tube ends. Use sampling-tube holders to reduce the hazard of
broken sampling-tube ends to the employee. All tubes should be
from the same lot.
The smaller section of the sampling
tube is used as a back-up and it is positioned nearest the
sampling pump. Attach the tube holder to the sampling pump with
flexible non-crimping tubing. Position the sampler in the
worker's breathing zone so that the sampling tube is in an
approximately vertical position with the inlet facing down
during sampling. Position the sampling pump, tube holder and
tubing so they do not impede work performance or safety.
Draw air to be sampled directly through the inlet of the
tube holder. The air being sampled should not pass through any
hose or tubing before entering the sampling tube.
After
sampling for the appropriate time, remove the sampling tube and
seal it with plastic end caps. Seal each sample end-to-end with
an OSHA-21 form as soon as possible.
Submit at least one
blank sample with each set of samples. Handle the blank sampler
in the same manner as the other samples except draw no air
through it.
Record sample air volume (liters), sampling
time (minutes) and sampling rate (mL/min) for each sample, along
with any potential interferences on the OSHA-91A form.
Submit the samples to the laboratory for analysis as
soon as possible after sampling. Store the samples in a
refrigerator if a delay is unavoidable. Ship any bulk samples
separate from the air samples.
2.3.2 SKC 575-002 Passive
Samplers (In general, follow the manufacturer's instructions.)
Remove the sampler from the clear, air-tight bag just
before sampling is to begin. CAUTION - The
monitor begins to sample immediately when it is removed from
this bag. Keep the O-ring, press-on cover, cover
retainer, port plugs and PTFE tube for later use.
Record
the start time on the sampler label and
on the Form OSHA-91A.
Attach the sampler to the worker
near his/her breathing zone with the perforations in the sampler
facing forward. Assure that the area directly in front of the
sampler is unobstructed throughout the sampling period.
At the end of the sampling period, immediately detach
the sampler from the worker and attach the cover with the O-ring
in place onto the sampler using the cover retainer. Visually
inspect the O-ring to be sure it is forming a proper seal around
the entire circumference of the sampler. Record the stop time on sampler label and on OSHA-91A
form.
Prepare a blank by removing an unused sampler from
its clear package and immediately attaching a cover with the
O-ring in place onto it.
Seal each sampler with an
OSHA-21 form.
Verify that the sampling times are
properly recorded on the OSHA-91A form for each sample. Also,
identify blank samples on this form.
Record the sampling site temperature and atmospheric
pressure on the Form OSHA-91A.
List any compounds
that could be considered potential interferences, especially
solvents, that are being used in the sampling area.
Submit the samples to the laboratory for analysis as
soon as possible after sampling. Store the samples in a
refrigerator if a delay is unavoidable. Include all port plugs
and PTFE tubes as they are used in the laboratory analyses. Ship
any bulk sample(s) in a container separate from the air samples.
2.3.3 3M OVMs (In general, follow the manufacturer's
instructions supplied with the samplers.)
The monitors
come individually sealed in small metal cans. Just before
sampling is to begin, remove the plastic lid from the can and
lift up on the revealed ring. Pull back on the ring to open the
can. Discard the metal top of the can and remove the monitor.
CAUTION - The monitor begins to sample
immediately when the can is unsealed.
Keep the
two closure caps with attached port plugs, cup and PTFE tubes in
the can for later use. Close the can with the plastic lid.
Record the start time on the
back of the monitor and on the OSHA-91A form.
Attach the
monitor to the worker near his/her breathing zone with the white
face forward. Assure that the area directly in front of the
sampler is unobstructed throughout the sampling period. Do not
remove the white film and ring from the monitor until the
sampling period is terminated.
At the end of the
sampling period, detach the monitor from the worker and remove
the white film and retaining ring. Immediately snap a closure
cap onto the primary (top) section of the monitor (where the
white film and ring were removed). It is critical that this step
be done as quickly as possible because the sampling rate is more
than five times greater without the white film in place. This
can be an important consideration, especially for short-term
sampling. Assure that the attached port plugs are placed firmly
into the port holes. The white film and ring can be discarded.
Record the stop time on the back of the
monitor and on the OSHA-91A form.
The following steps
should be performed in a low background (uncontaminated) area
for a set of monitors as soon as possible after sampling.
Prepare a blank by removing a new sampler from its can.
Immediately remove the white film and ring and then immediately
attach a closure cap onto the unused monitor.
For each
monitor (one at a time), separate the primary (top) and
secondary (bottom) sections of the monitor using the edge of a
coin as a pry.
Securely snap a cup onto the bottom of
the primary section.
Snap a closure cap onto the
secondary section of the monitor and assure that the attached
port plugs are placed firmly into the port holes.
Return
the sampler sections with closure caps and cup in place to the
metal can containing the PTFE tubes (which will be used by the
laboratory). Close the can with the plastic lid, and seal it
with an OSHA-21 form.
Verify that the sampling times are
properly recorded on OSHA-91A form for each sample. Also,
identify blank samples on this form.
Record the sampling site temperature and atmospheric
pressure on the OSHA-91A form.
List any compounds
that could be considered potential interferences, especially
solvents, that are being used in the sampling area.
Submit the samples to the laboratory for analysis as
soon as possible after sampling. Store the samples in a
refrigerator if a delay is unavoidable. Ship any bulk sample
separate from the air samples. 2.4 Sampler capacity
(Section
4.7)
2.4.1 The sampling capacity of the front sections of
SKC Anasorb CMS sampling tubes was tested by sampling a
dynamically generated test atmosphere of MEK and MIBK (1131
mg/m3 or 384 ppm MEK, and 774 mg/m3, or
189 ppm MIBK) at an absolute humidity of 13.9 milligrams of
water per liter of air (74% relative humidity at 22°C). The
samples were collected at 50 mL/min. The 5% breakthrough
sampling time for MEK was determined to be 300 min. No
breakthrough of MIBK was observed, even after samples were
collected for 600 min.
Table
2.4.2
Sampling Rates (mL/min)
at 760 mmHg and 298.2
K |
|
| |
MEK |
MIBK |
|
SKC 575-002
3M 3520 |
16.88
32.59 |
13.62
27.00 |
| 2.4.2 The sampling
rate and capacity of SKC 575-002 Passive Samplers and of 3M 3520
OVMs were determined by sampling from atmospheres of MEK and
MIBK at about two times their respective target concentrations
and at an absolute humidity of 16.4 milligrams of water per
liter of air (about 80% relative humidity at 23°C) for
increasing time intervals. The sampling rates are shown in Table
2.4.2. A recommenced sampling time of 240 min was obtained from
this test. 2.5 Extraction
efficiency (Section
4.8)
It is the responsibility of each analytical
laboratory to determine extraction efficiency in-house because the
adsorbent material, internal standard, reagents and laboratory
techniques could be different than those used in this evaluation
and they could influence analytical results.
The mean
extraction efficiencies of MEK and MIBK from dry media over the
range of the RQL to 2 times the target concentrations are shown in
Table 2.5. The extraction efficiency for MEK was affected by the
presence of water. This effect was caused by the instability of
MEK on wet carbon-based sorbents. MIBK was not affected by the
presence of water.
Table
2.5
Extraction Efficiency Summary |
|
| |
MEK
|
|
MIBK
|
| medium |
RQL(µg) |
2×(µg) |
EE(%) |
|
RQL(µg) |
2×(µg) |
EE(%) |
|
Anasorb CMS
SKC 575-002
3M 3520
OVM |
0.81
1.28
0.76 |
14250
4750
9500 |
100.3
92.2
98.0 |
|
0.42
1.27
0.94 |
9753
2601
5201 |
102.3
92.9
96.5 |
|
Extracted
MEK samples with punctured septa gave results more than 10% lower
than MEK samples with intact septa upon standing in an autosampler
rack for a day. The loss was attributed to volatility of MEK, room
temperature, and septa condition rather than chemical reactivity
of MEK.
Extracted MEK samples with punctured septa
remained stable for 12 h. Extracted MIBK samples with punctured
septa remained stable for at least a day. Extracted samples for
both MEK and MIBK with intact septa remained stable for at least a
day.
2.6 Recommended sampling time and sampling rate
2.6.1 SKC Anasorb CMS sampling tubes
Sample
for up to 240 min at 50 mL/min (12 L) when using SKC Anasorb CMS
sampling tubes to collect TWA (long-term) samples.
Sample for 5 min at 50 mL/min (0.25 L) when using SKC
Anasorb CMS sampling tubes to collect ceiling (short-term)
samples.
When short-term samples are collected, the air
concentration equivalent to the reliable quantitation limit
becomes larger. For example, the reliable quantitation limit for
SKC Anasorb CMS sampling tubes is 1.1 ppm (3.25
mg/m3) for MEK when 0.25 L is sampled.
2.6.2
SKC 575-002 Passive Samplers and 3M 3520 OVMs
Table
2.6.2
Sampling Rates(mL/min) at
760 mmHg and 298.2
K |
|
| |
MEK |
MIBK |
|
SKC 575-002
3M 3520 |
16.88
32.59 |
13.62
27.00 |
|
Sample for up
to 240 min when using SKC 575-002 Passive Samplers and 3M 3520
OVMs to collect TWA (long-term) samples.
Sample for 5
min when using SKC 575-002 Passive Samplers and 3M 3520 OVMs to
collect ceiling (short-term) samples.
When short-term
samples are collected, the air concentration equivalent to the
reliable quantitation limit becomes larger. For example, the
reliable quantitation limit for 3M 3520 OVMs is 1.6 ppm (4.70
mg/m3) for MEK when 0.163 L is sampled.
2.7 Interferences, sampling (Section
4.9)
2.7.1 Active sampler
Retention
efficiency
Table
2.7.1.1
Mean Recovery |
|
| MEK(%) |
MIBK(%) |
|
| 95.6 |
97.2 |
|
Six SKC
Anasorb CMS sampling tubes were used to sample a test atmosphere
containing two times the target concentrations of MEK and of
MIBK for one hour. Three samples were removed and analyzed after
the initial one hour, and the remaining three were used to
sample contaminant-free air for an additional three hours (four
hours total). The absolute humidity of the test atmosphere was
14.6 milligrams of water per liter of air (77.8% relative
humidity at 21.4°C). The percent of the four-hour sample means
relative to the one-hour sample means are shown in Table
2.7.1.1. MEK and MIBK were efficiently retained following
collection.
Low humidity
Three SKC Anasorb CMS
sampling tubes were used to sample a test atmosphere containing
two times the target concentrations of MEK and of MIBK. The
absolute humidity of the test atmosphere was 2.7 milligrams of
water per liter of air (13.2% relative humidity at 22.6°C). The
recovery for all samples was more than 95.1% of theoretical for
MEK and 98.2% of theoretical for MIBK. Low humidity had no
significant effect on recovery.
Low concentration
Three SKC Anasorb CMS sampling tubes were used to sample
a test atmosphere containing 0.1 times the target concentrations
of MEK and of MIBK. The absolute humidity of the test atmosphere
was 15.5 milligrams of water per liter of air (79.7% relative
humidity at 22.3°C). The recovery for all samples was more than
94.9% of theoretical for MEK and 101.1% of theoretical for MIBK.
Low concentration had no significant effect on recovery.
Sampling interferences
Three Anasorb CMS
sampling tubes were used to sample a test atmosphere containing
one times the target concentrations of MEK and of MIBK; and
553.3 mg/m3 of acetone, 253.7 mg/m3 of
isopropyl alcohol, 190.1 mg/m3 of toluene, 92.5
mg/m3 of xylene isomers, and 16.3 mg/m3 of
ethyl benzene. The absolute humidity was 15.6 milligrams of
water per liter of air (79.0% relative humidity at 22.3°C). The
recovery for all samples was more than 99.0% of theoretical for
MEK and 102.2% of theoretical for MIBK. The sampling
interferences had no significant effect on recovery.
2.7.2 Diffusive samplers
Reverse
diffusion
Table
2.7.2.1
Mean Recovery |
|
SKC 575-002
|
3M 3520
|
| MEK(%) |
MIBK(%) |
MEK(%) |
MIBK(%) |
|
| 99.3 |
100.0 |
96.0 |
98.6 |
|
Six SKC
575-002 and six 3M 3520 samplers were used to test for reverse
diffusion by first exposing the samplers to a test atmosphere
containing two times the target concentrations of MEK and of
MIBK for one hour. Three of each sampler were analyzed after the
initial exposure, and the remaining three of each sampler were
exposed to contaminant-free air for an additional three hours
(four hours total). The absolute humidity of the test atmosphere
was 14.6 milligrams of water per liter of air (77.8% relative
humidity at 21.4°C). The percent of the means of the four hour
samples relative to the one hour sample means are shown in Table
2.7.2.1. Reverse diffusion was not significant.
Low
humidity
Table
2.7.2.2
Low Humidity (% recovery) |
|
| analyte |
SKC 575-002 |
3M 3520 |
|
MEK
MIBK |
92.2
102.5 |
92.9
101.0 |
|
Three SKC
and three 3M diffusive samplers were used to sample a test
atmosphere containing two times the target concentrations of MEK
and of MIBK. The absolute humidity of the test atmosphere was
2.7 milligrams of water per liter of air (13.2% relative
humidity at 22.6°C). The lowest recoveries for all samplers are
shown in Table 2.7.2.2. Low humidity had no significant effect
on recovery.
Low concentration
Table
2.7.2.3
Low Concentration (% recovery) |
|
| analyte |
SKC 575-002 |
3M 3520 |
|
MEK
MIBK |
90.3
96.8 |
89.8
95.0 |
|
Three SKC
and three 3M diffusive samplers were used to sample a test
atmosphere containing 0.1 times the target concentrations of MEK
and of MIBK. The absolute humidity of the test atmosphere was
15.5 milligrams of water per liter of air (79.7% relative
humidity at 22.3°C). The lowest recoveries for all samplers are
shown in Table 2.7.2.3. Low concentration had no significant
effect on recovery.
Sampling interferences
Table
2.7.2.4
Sampling Interferences (% recovery) |
|
| analyte |
SKC 575-002 |
3M 3520 |
|
MEK
MIBK |
97.6
102.2 |
93.5
101.8 |
|
Three SKC
and three 3M diffusive samplers were used to sample a test
atmosphere containing one times the target concentrations of MEK
and of MIBK; and 553.3 mg/m3 of acetone, 253.7
mg/m3 of isopropyl alcohol, 190.1 mg/m3 of
toluene, 92.5 mg/m3 of xylene isomers, and 16.3
mg/m3 of ethyl benzene. The absolute humidity was
15.6 milligrams of water per liter of air (79.0% relative
humidity at 22.3°C). The lowest recoveries for all samplers are
shown in Table 2.7.2.4. The sampling interferences had no
significant effect on recovery.
3. Analytical
Procedure
Adhere to the rules set down
in your Chemical Hygiene Plan10.
Avoid skin contact and inhalation of all chemicals and review all
appropriate MSDSs before beginning this analytical procedure.
3.1 Apparatus
3.1.1 A GC equipped with a flame ionization detector
(FID). A Hewlett-Packard Model 5890 Series II GC equipped with a
ChemStation, an automatic sample injector, and an FID were used
in this evaluation.
3.1.2 A GC column capable of
separating MEK and MIBK from the extraction solvent, internal
standards, and potential interferences. A J&W Scientific
60-m × 0.32-mm i.d. DB-Wax (0.5-µm df) capillary column was used
in this evaluation.
3.1.3 An electronic integrator or
other suitable means of measuring GC detector response. A Waters
Millennium Chromatography Manager system was used in this
evaluation.
3.1.4 Two and four-milliliter glass vials
with PTFE-lined septum caps.
3.1.5 One and
two-milliliter volumetric pipets.
3.1.6 An SKC
Desorption Shaker with rack (226D-03K) was used to extract SKC
575-002 Passive Samplers in this evaluation. 3.2
Reagents
3.2.1 2-Butanone (methyl ethyl ketone, MEK) [CAS no.
78-93-3], reagent grade or better. The MEK used in this
evaluation was 99+% A.C.S. Reagent grade (lot no. 11619CX)
purchased from Aldrich (Milwaukee, WI).
3.2.2 Hexone
(methyl isobutyl ketone, MIBK) [CAS no. 108-10-1], reagent grade
or better. The MIBK used in this evaluation was Analytical
Reagent grade (lot no. CVT) purchased from Mallinckrodt (St.
Louis, MO)
3.2.3 Carbon disulfide (CS2), [CAS
no. 75-15-0], reagent grade or better. The carbon disulfide used
in this evaluation was 99.9+% low benzene content grade (lot no.
1054JQ) purchased from Aldrich (Milwaukee, WI).
3.2.4
N,N-Dimethyl formamide (DMF) [CAS no.
68-12-2], reagent grade or better. The DMF used in this
evaluation was Certified A.C.S. grade (lot no. 902902) purchased
from Fisher (Fair Lawn, NJ).
3.2.5 1-Phenylhexane
(hexylbenzene) [CAS no. 1077-16-3], reagent grade or better. The
1-phenylhexane used in this evaluation was 97% reagent grade
(lot no. 03006PZ) purchased from Aldrich (Milwaukee, WI).
3.2.6 The extraction solvent used for this evaluation
consisted of 1% DMF in CS2. 1-Phenylhexane (1 µL/mL)
was added to the solution for use as an internal standard. Other
internal standards can be used provided they are fully tested.
3.3 Standard preparation
3.3.1 Prepare concentrated stock standards by
weighing 3 mL of MEK and/or 2 mL of MIBK into a 5-mL volumetric
flask, and diluting to the mark with CS2. Obviously,
no CS2 addition is necessary if both MEK and MIBK are
added to the same flask. Prepare working analytical standards by
injecting microliter amounts of concentrated stock standards
into vials containing either 1 or 2 mL of extracting solution
delivered from the same dispenser used to extract samples. For
example, to prepare a target level standard to analyze SKC
Anasorb CMS sampling tubes, inject 15 µL of a stock solution
containing 480 mg/mL of MEK and 320 mg/mL MIBK into 1 mL of
extracting solution.
3.3.2 Bracket sample concentrations
with standard concentrations. If, upon analysis, sample
concentrations fall outside the range of prepared standards,
prepare and analyze additional standards to confirm instrument
response, or dilute high samples with extraction solvent and
reanalyze the diluted samples. 3.4 Sample preparation
3.4.1 SKC Anasorb CMS sampling tubes
Remove
the plastic end caps from the sample tube and carefully transfer
each section of the adsorbent to separate 2-mL vials. Discard
the glass tube and glass wool plugs.
Add 1.0 mL of
extracting solution to each vial and immediately seal the vials
with polytetrafluoroethylene-lined caps.
Shake the vials
vigorously several times during the 60 min extraction time.
3.4.2 SKC 575-002 Passive Samplers (In general, follow
the manufacturer's instructions.)
Cut off the ends of
the two protruding tubes (ports) of each sampler with a razor
blade or sharp knife.
Slowly and carefully add 2.0 mL of
extraction solvent through the protruding tube (port) nearest
the outside edge of the sampler.
Immediately insert
plugs into the ports.
Mount the samplers in the sampler
rack (SKC Cat. No. 226-04-5) of a specialized shaker (SKC Cat.
No. 226D-03-1) and shake the samplers for 1 hour.
Do not
leave the extracted sample in the sampler. Transfer each
extracted sample by removing the plugs from the sampler ports,
firmly inserting the tapered end of a supplied PTFE tube into
the outer port, and carefully pouring the solution through the
PTFE tube into a labeled autosampler vial. Immediately cap each
vial.
3.4.3 3M 3520 OVMs (In general, follow the
manufacturer's instructions.)
Remove both sampler
sections from the metal cans, along with the sections of PTFE
tubing. Assure that the closure caps are firmly snapped to the
primary and secondary sections of all the samplers. Also assure
that all cap plugs are firmly seated in the cap ports. Any
deviations must be noted.
Prepare one section of the
sampler at a time by temporarily removing the cap plugs from the
ports and adding 2.0 mL of extraction solvent through the center
port.
Allow the sampler sections to extract for one
hour. Apply gentle agitation to the sampler sections,
periodically, during the extraction period.
Do not leave
the extracted sample in the sampler. Transfer the solution from
each sampler section by removing both plugs from the ports,
firmly inserting a decanting spout (a small section of PTFE
tubing) into the outer port and carefully pouring the liquid
through the spout into a labeled autosampler vial. Immediately
cap each vial. 3.5 Analysis
3.5.1 Analytical conditions
GC conditions
|
|
| column temperature: |
initial 40°C, hold 1 min,
program at 4°C/min to 140°C, hold until column is
clear. |
| zone temperatures: |
220°C (injector)
220°C
(detector) |
| run time: |
26 min |
| column gas flow: |
4.9 mL/min (hydrogen) |
| septum purge: |
3.3 mL/min (hydrogen) |
| injection size: |
1.0 µL (26:1 split) |
| column: |
60-m × 0.32-mm i.d.
capillary J&W DB-Wax (0.5-µm df) |
| retention times: |
4.1 min (MEK)
6.3
min (MIBK)
23.4 min (1-phenylhexane) |
FID conditions
|
| hydrogen flow: |
35 mL/min |
| air flow: |
450 mL/min |
| nitrogen makeup flow: |
37
mL/min |

Figure 3.5.1. Chromatogram obtained at
the target concentrations with the recommended
conditions.
(1- CS2; 2- MEK; 3- MIBK; 4-
DMF;
5-
1-phenylhexane) |
3.5.2 An internal standard (ISTD) calibration
method is used. A calibration curve can be constructed by
plotting ISTD-corrected response of standard injections versus
micrograms of analyte per sample. Bracket the samples with
freshly prepared analytical standards over a range of
concentrations.
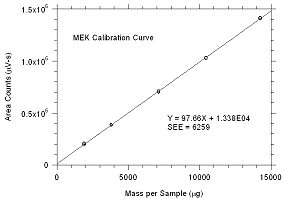
Figure
3.5.2.1. Calibration curve for MEK |
|

Figure
3.5.2.2. Calibration curve for
MIBK |
3.6 Interferences (analytical)
3.6.1 Any compound that produces an FID response and
has a similar retention time as the analyte or internal standard
is a potential interference. If any potential interferences were
reported, they should be considered before samples are
extracted. Generally, chromatographic conditions can be altered
to separate an interference from the analyte.
3.6.2 When
necessary, the identity of an analyte peak can be confirmed with
additional analytical data (Section
4.9). 3.7 Calculations
3.7.1 SKC Anasorb CMS sampling tubes
The
amount of MEK and/or MIBK per sample is obtained from the
appropriate calibration curve in terms of micrograms per sample,
uncorrected for extraction efficiency. The back section is
analyzed primarily to determine the extent of sampler
saturation. If any analyte is found on the back section, it is
added to the amount on the front section. This total amount is
then corrected by subtracting the total amount (if any) found on
the blank. The air concentration is calculated using the
following formulas.
|
|
| where: |
CM |
is concentration by weight
(mg/m3) |
| M |
is micrograms per sample |
| V |
is liters of air sampled |
| EE |
is extraction efficiency, in
decimal form | |
| |
|
|
| where: |
CV |
is concentration by volume
(ppm)
|
| VM |
is molar volume at 25°C and 760
mmHg (NTP) |
| CM |
is concentration by weight |
| Mr |
is molecular weight (MEK =
72.10,
MIBK =
100.16) | |
3.7.2 Diffusive samplers
Table
3.7.2
Sampling Rates (mL/min)
at 760 mmHg and 298.2
K (NTP) |
|
| |
MEK |
MIBK |
|
SKC 575-002
3M 3520 |
16.88
32.59 |
13.62
27.00 |
|
The amount
of MEK and/or MIBK for the samples is obtained from the
appropriate calibration curve in terms of micrograms per sample,
uncorrected for extraction efficiency. The 3M 3520 is a
two-section sampler and the back section is analyzed primarily
to determine the extent of sampler saturation. If any analyte is
found on the back section, the amount is multiplied by 2.2 (as
per manufacturer's instructions) and then added to the amount on
the front section. The total amount is then corrected by
subtracting the total amount (if any) found on the blank. The
air concentration is calculated using the following formulas.
| RSS |
= |
RNTP |
( |
Tss |
) |
3/2 |
( |
PNTP |
) |
|
|
| TNTP |
PSS | |
| where |
RSS |
is the sampling
rate at sampling site |
| RNTP |
is the
sampling rate at NTP |
| TSS |
is the
sampling site temperature in K |
| TNTP |
is 298.2
K |
| PSS |
is the
sampling site pressure in mmHg |
| PNTP |
is 760
mmHg | |
| |
|
|
| where |
CM |
is concentration by weight
(mg/m3) |
| M |
is micrograms per sample |
| RSS |
is the sampling rate at the
sampling site |
| t |
is the sampling time |
| EE |
is extraction efficiency, in
decimal form | |
| |
|
|
| where |
CV |
is concentration by
volume (ppm) |
| VM |
is molar volume at
NTP |
| CM |
is concentration by
weight |
| Mr |
is molecular weight (MEK
= 72.10,
MIBK =
100.16) | |
If the sampling site temperature is not provided,
assume that it is 22.2 °C. If the sampling site atmospheric
pressure is not given, calculate an approximate value based on
the sampling site elevation from the following equation.
| PSS =
AE2 - BE + 760.0 |
| where |
PSS |
is the approximate atmospheric
pressure |
| E |
is the sampling site elevation,
ft |
| A |
is 3.887×10-7
mmHg/ft2 |
| B |
is 0.02748
mmHg/ft | |
4. Backup Data
General background information about the
determination of detection limits and precision of the overall
procedure is found in the "Evaluation Guidelines for Air Sampling
Methods Utilizing Chromatography Analysis"11.
The Guidelines define analytical parameters, specific laboratory
tests, statistical calculations and acceptance criteria.
4.1 Detection
limit of the analytical procedure (DLAP)
DLAP is measured as the mass of analyte introduced
onto the chromatographic column. Ten analytical standards were
prepared with equal increments with the highest standard
containing 475 ng/mL of MEK and 553 ng/mL of MIBK. These are
concentrations that would produce peaks approximately 10 times
the response of a reagent blank near the elution times of the
analytes. These standards, and the reagent blank were analyzed
with the recommended analytical parameters (1-µL injection with
a 26:1 split), and the data obtained were used to determine the
required parameters (standard error of estimate and slope) for
the calculation of the DLAP. The DLAP for MEK was 4.90 pg and it
was 3.13 pg for MIBK.
Table
4.1.1
Detection Limit
of the Analytical Procedure
for MEK |
|
concentration
(ng/mL) |
mass on column
(pg) |
area counts
(µV-s) |
|
0
48
95
142
190
238
285
332
380
428
475 |
0
1.8
3.6
5.5
7.3
9.2
11.0
12.8
14.6
16.5
18.3 |
0
16
28
35
43
60
40
70
74
83
96 |
| |

Figure
4.1.1 Plot of data used to determine the DLAP for MEK. |
| |
Table
4.1.2
Detection Limit
of the Analytical Procedure
for MIBK |
|
concentration
(ng/mL) |
mass on column
(pg) |
area counts
(µV-s) |
|
0
33
98
163
228
293
358
390
423
488
553 |
0
1.3
3.8
6.3
8.8
11.3
13.8
15.0
16.3
18.8
21.3 |
0
12
23
45
55
68
90
90
92
99
132 |
| |
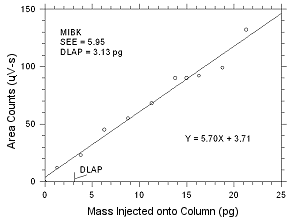
Figure
4.1.2 Plot of data used to determine the DLAP for
MIBK. |
4.2 Detection
limit of the overall procedure (DLOP) and reliable quantitation
limit (RQL)
DLOP is measured as mass per sample and
expressed as equivalent air concentrations, based on the
recommended sampling parameters. Ten samplers were spiked with
descending increments of analyte. The highest amount shown in
the following tables is the amount spiked on a sampler that
would produce a peak approximately 10 times the response of a
sample blank. These spiked samplers, and the sample blanks were
analyzed with the recommended analytical parameters, and the
data obtained used to calculate the required parameters
(standard error of estimate and the slope) for the calculation
of the DLOP.
Table
4.2
Detection Limits of the Overall Procedure |
|
| |
MEK
|
|
MIBK
|
| sampler |
ng |
ppb |
µg/m3 |
|
ng |
ppb |
µg/m3 |
|
SKC CMS
SKC Anasorb 747
SKC
575-002
3M 3520 |
244
291
388
230 |
7
8
33
10 |
20
24
96
29 |
|
129
329
376
281 |
3
7
28
11 |
11
27
115
43 |
|
Table
4.2.1
DLOP/RQL for MEK Collected
on SKC CMS
Sampling Tubes |
|
mass per sample
(ng) |
area counts
(µV-s) |
|
0
48
142
238
332
428
522
570
618
713
808 |
0
26
14
31
44
47
54
58
66
66
94 |
| |
|
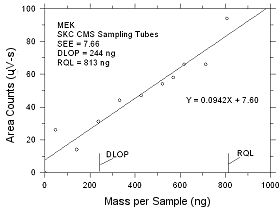
Figure
4.2.1 Plot of data to determine the DLOP/RQL for MEK collected
on SKC CMS sampling tubes. |
| |
Table
4.2.2
DLOP/RQL for MIBK Collected
on SKC CMS
Sampling Tubes |
|
mass per sample
(ng) |
area counts
(µV-s) |
|
0
65
130
195
260
325
358
390
455
520
553 |
0
0
16
22
28
32
40
43
66
64
61 |
| |
|

Figure
4.2.2 Plot of data to determine the DLOP/RQL for MIBK
collected on SKC CMS sampling tubes. |
| |
Table
4.2.3
DLOP/RQL for MEK Collected
on SKC Anasorb
747 Sampling Tubes |
|
mass per sample
(ng) |
area counts
(µV-s) |
|
0
190
380
570
665
760
855
1045
1235
1425
1615 |
0
11
18
25
31
36
42
46
57
68
93 |
| |
|
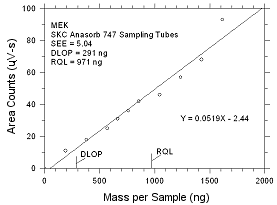
Figure
4.2.3 Plot of data to determine the DLOP/RQL for MEK collected
on SKC Anasorb 747 sampling tubes. |
| |
Table
4.2.4
DLOP/RQL for MIBK Collected
on SKC Anasorb
747 Sampling Tubes |
|
mass per sample
(ng) |
area counts
(µV-s) |
|
0
130
260
390
455
520
585
715
845
975
1105 |
0
13
24
40
33
35
42
53
57
76
110 |
| |
|
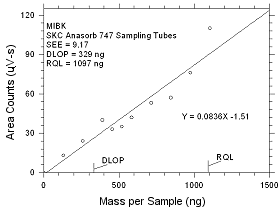
Figure
4.2.4 Plot of data to determine the DLOP/RQL for MIBK
collected on SKC Anasorb 747 sampling tubes. |
| |
Table
4.2.5
DLOP/RQL for MEK Collected
on SKC 575-002
Passive Samplers |
|
mass per sample
(ng) |
area counts
(µV-s) |
|
0
190
38
570
665
760
855
1045
1235
1425
1615 |
0
24
34
40
47
46
46
59
70
90
82 |
| |
|
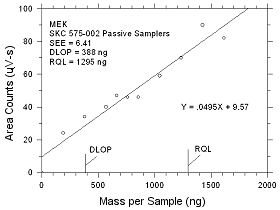
Figure
4.2.5 Plot of data to determine the DLOP/RQL for MEK collected
on SKC 575-002 Passive Samplers. |
| |
Table
4.2.6
DLOP/RQL for MIBK Collected
on SKC 575-002
Passive Samplers |
|
mass per sample
(ng) |
area counts
(µV-s) |
|
0
130
260
390
455
520
585
715
845
975
1105 |
0
11
21
28
29
40
39
66
77
55
79 |
| |
|
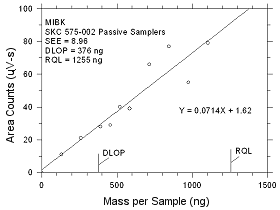
Figure
4.2.6 Plot of data to determine the DLOP/RQL for MIBK
collected on SKC 575-002 Passive Samplers. |
| |
Table
4.2.7
DLOP/RQL for MEK
Collected on 3M 3520
OVMs |
|
mass per sample
(ng) |
area counts
(µV-s) |
|
0
95
285
475
665
855
1045
1140
1235
1425
1615 |
0
6
24
29
42
49
58
65
79
80
86 |
| |
|

Figure
4.2.7 Plot of data to determine the DLOP/RQL for MEK collected
on 3M 3520 OVMs. |
| |
Table
4.2.8
DLOP/RQL for MIBK
Collected on 3M 3520
OVMs |
|
mass per sample
(ng) |
area counts
(µV-s) |
|
0
65
195
325
455
585
715
780
845
975
1105 |
0
4
14
18
24
44
43
48
54
81
70 |
| |
|

Figure
4.2.8 Plot of data to determine the DLOP/RQL for MIBK
collected on 3M 3520 OVMs. |
The RQL is considered the lower
limit for precise quantitative measurements. It is determined
from the regression line parameters obtained for calculation of
the DLOP, providing the extraction efficiency (EE) is 75% to 125%.
Table
4.2.9
Reliable Quantitation Limits |
|
| |
MEK
|
|
MIBK
|
| sampler |
ng |
ppb |
µg/m3 |
EE |
|
ng |
ppb |
µg/m3 |
EE |
|
SKC CMS
SKC Anasorb 747
SKC
575-002
3M 3520 |
813
971
1295
766 |
23
27
109
33 |
68
81
320
98 |
98.5
101.5
90.4
105.3 |
|
430
1097
1255
937 |
9
22
94
35 |
36
91
384
144 |
111.7
98.2
81.0
86.4 |
|

Figure
4.2.9 Chromatogram of the RQL for MEK extracted from SKC
Anasorb CMS sampling tubes. Peak 1 is MEK. |
|
Figure
4.2.10 Chromatogram of the RQL for MIBK extracted from SKC
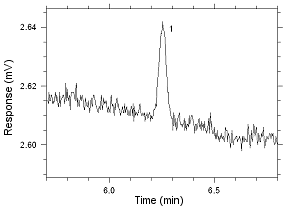
Anasorb CMS sampling tubes. Peak 1 is MIBK. |
|
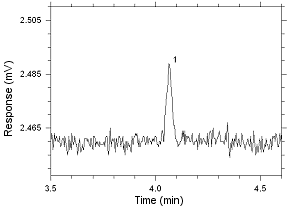
Figure
4.2.11 Chromatogram of the RQL for MEK extracted from SKC
Anasorb 747 sampling tubes. Peak 1 is MEK. |
|
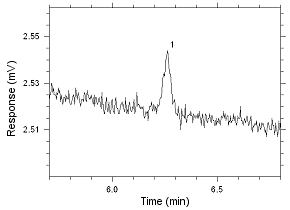
Figure
4.2.12 Chromatogram of the RQL for MIBK extracted from SKC
Anasorb 747 sampling tubes. Peak 1 is MIBK. |
|
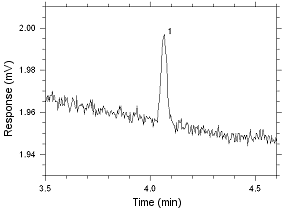
Figure
4.2.13 Chromatogram of the RQL for MEK extracted from SKC
575-002 Passive Samplers. Peak 1 is MEK. |
|
Figure
4.2.14 Chromatogram of the RQL for MIBK extracted from SKC
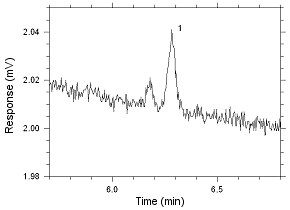
575-002 Passive Samplers. Peak 1 is MIBK. |
|
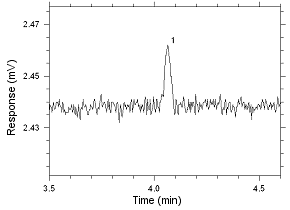
Figure
4.2.15 Chromatogram of the RQL for MEK extracted from 3M 3520
OVM. Peak 1 is MEK. |
|

Figure
4.2.16 Chromatogram of the RQL for MIBK extracted from 3M 3520
OVM. Peak 1 is MIBK. |
4.3 Instrument Calibration
The standard errors of estimate were determined from the
linear regressions of data points from standards over a range
that covers 0.25 to 2 times the target concentrations for SKC
CMS sampling tubes. This was the sampler with the highest mass
loadings. Calibration curves were constructed and shown in
Section 3.5.2 from the injections of the fifteen standards. The
standard errors of estimate are 64 µg/sample for MEK and 47
µg/sample for MIBK.
Table
4.3.1
Instrument Calibration for MEK |
|
standard concn
(µg/sample) |
|
area counts
(µV·s) |
|
1900
3800
7125
10450
14250 |
|
209059
382186
711627
1030541
1410615 |
203442
383631
702855
1028461
1414645 |
194603
389559
701446
1025964
1406129 |
|
Table 4.3.2
Instrument Calibration for
MIBK |
|
standard
concn
(µg/sample) |
|
area
counts
(µV·s) |
|
1300
2601
4876
7152
9753 |
|
171450
313431
584366
845491
1157297 |
166556
313539
575873
842971
1161587 |
158962
319156
576681
839986
1153280 |
|
4.4 Precision (overall procedure)
4.4.1 SKC CMS sampling tubes
The precision
at the 95% confidence level is obtained by multiplying the
standard error of estimate by 1.96 (the z-statistic from the
standard normal distribution at the 95% confidence level). In
Section
4.5, 95% confidence intervals are drawn about their
respective regression lines in the storage graph figures. Each
precision includes an additional 5% for sampling error. The
precisions of the overall procedure at the 95% confidence
interval for the ambient temperature 15-day storage tests (at
the target concentration) for MEK and for MIBK were ±11.7% and
±11.5%, respectively. These values are from the standard error
of estimates shown in Figures
4.5.1.1 and 4.5.1.3.
4.4.2 Diffusive samplers
The precisions of the overall
procedure at the 95% confidence level for the ambient
temperature 15-day storage tests (at the target concentration)
are shown in Table 4.4.2. There are different values given,
depending on whether both, either, or neither temperature
(T) or atmospheric pressure (P) at the sampling site are known. If the
sampling site temperature is unknown, it is assumed to be 22.2
± 15°C (72 ± 27°F) and a variability of ±7.7% is included. If
the atmospheric pressure is not known, it is estimated from
the sampling site elevation and a variability of ±3% is
included. Each 3M precision value includes an additional 7.4%
for sampling rate variation, and each SKC value an additional
8.7%, also for sampling rate variation 12.
Table
4.4.2
Standard Error of Estimate and Precision
of the
overall procedure for Diffusive Samplers |
|
| |
SKC 575-002
Passive
Sampler
|
|
3M 3520 OVM
|
| |
MEK |
MIBK |
|
MEK |
MIBK |
known
condition |
SEE (%) |
precision (±%) |
SEE (%) |
precision (±%) |
|
SEE (%) |
precision (±%) |
SEE (%) |
precision (±%) |
|
both T&P
only T
only P
neither T nor P |
9.12
9.60
11.94
12.30 |
17.8
18.7
23.3
24.1 |
9.05
9.53
11.88
12.26 |
17.7
18.7
23.3
24.0 |
|
8.32
8.84
11.34
11.73 |
16.3
17.3
22.2
23.0 |
8.03
8.57
11.13
11.52 |
15.7
16.8
21.8
22.6 |
|
4.5 Storage
tests
4.5.1 Active samples
Storage samples for
MEK and MIBK were generated by sampling controlled test
atmospheres with SKC CMS and with SKC Anasorb 747 sampling
tubes at 50 mL/min for four hours. The analyte concentrations
of the test atmospheres were the target concentrations with an
absolute humidity of 14.3 milligrams of water per liter of air
(77.2% at 21.1°C). Thirty-three storage samples were collected
with each sampler. Three of each sampler were analyzed on the
day of generation. Fifteen of each sampler were stored at
reduced temperature (about 4°C) and the other fifteen were
stored in a closed drawer at ambient temperature (about 22°C).
Three samples were selected from each of the storage sets and
analyzed at 2-4 day intervals. Sample results were not
corrected for extraction efficiency.
Table
4.5.1.1
Storage Tests for MEK on
SKC CMS Sampling
Tubes |
|
time
(days) |
ambient storage
recovery
(%) |
|
refrigerated
storage
recovery (%) |
|
0
2
5
8
12
15 |
95.0
90.7
88.2
92.3
92.2
85.8 |
94.4
89.2
83.7
89.7
86.3
80.4 |
90.8
96.4
88.0
91.5
87.3
82.7 |
|
95.0
96.3
93.0
96.2
94.0
92.6 |
94.4
95.9
92.7
94.3
94.1
94.2 |
90.8
96.4
92.0
95.1
93.3
88.8 |
|
Table
4.5.1.2
Storage Tests for MIBK on
SKC CMS Sampling
Tubes |
|
time
(days) |
ambient storage
recovery
(%) |
|
refrigerated
storage
recovery (%) |
|
0
2
5
8
12
15 |
96.1
92.7
91.0
96.6
96.7
95.1 |
95.3
91.4
89.7
94.6
94.4
88.5 |
92.6
97.5
88.0
96.5
95.1
90.6 |
|
96.1
96.3
95.2
96.3
95.3
95.5 |
95.3
96.1
94.6
96.4
95.9
97.2 |
92.6
95.9
93.1
97.0
96.0
96.0 |
|
Table
4.5.1.3
Storage Tests for MEK on
SKC Anasorb 747
Sampling Tubes |
|
time
(days) |
ambient storage
recovery
(%) |
|
refrigerated
storage
recovery (%) |
|
0
2
5
8
12
15 |
89.3
82.8
75.9
76.7
74.2
73.3 |
90.7
82.6
77.1
76.6
73.2
74.0 |
90.8
84.0
78.4
76.9
70.6
70.4 |
|
89.3
88.9
86.5
82.3
81.2
79.3 |
90.7
88.5
87.1
81.5
80.5
81.1 |
90.8
87.1
85.8
83.1
78.7
80.4 |
|
Table
4.5.1.4
Storage Tests for MIBK on
SKC Anasorb 747
Sampling Tubes |
|
time
(days) |
ambient storage
recovery
(%) |
|
refrigerated
storage
recovery (%) |
|
0
2
5
8
12
15 |
93.6
93.8
91.8
93.4
92.4
93.5 |
93.9
93.4
93.8
93.7
91.5
93.0 |
94.4
94.6
92.9
93.3
88.5
89.2 |
|
93.6
93.1
93.9
92.4
92.9
92.4 |
93.9
94.5
91.2
92.2
93.1
93.6 |
94.4
92.9
93.0
91.6
91.3
93.8 |
|

Figure
4.5.1.1 Ambient Storage for MEK collected on SKC CMS sampling
tubes. |
|
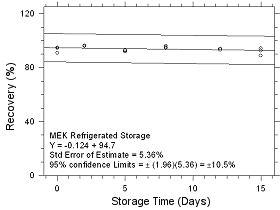
Figure
4.5.1.2 Refrigerated Storage for MEK collected on SKC CMS
sampling tubes. |
|

Figure
4.5.1.3 Ambient Storage for MIBK collected on SKC CMS sampling
tubes. |
|
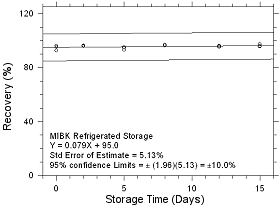
Figure
4.5.1.4 Refrigerated Storage for MIBK collected on SKC CMS
sampling tubes. |
|

Figure
4.5.1.5 Ambient Storage for MEK collected on SKC Ansasorb 747
sampling tubes. |
|
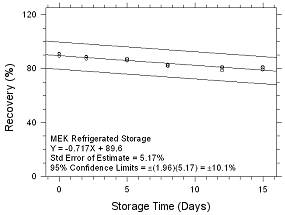
Figure
4.5.1.6 Refrigerated Storage for MEK collected on SKC Anasorb
747 sampling tubes. |
|
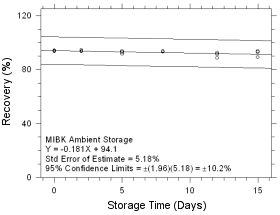
Figure
4.5.1.7 Ambient Storage for MIBK collected on SKC Anasorb 747
sampling tubes. |
|

Figure
4.5.1.8 Refrigerated Storage for MIBK collected on SKC Anasorb
747 sampling tubes. |
4.5.2 Diffusive samplers
Storage samples for MEK and MIBK were generated by
sampling controlled test atmospheres with SKC 575-002 Passive
Samplers and with 3M 3520 OVMs for four hours. The analyte
concentrations of the test atmospheres were the target
concentrations with an absolute humidity of 14.3 milligrams of
water per liter of air (77.2% at 21°C). Thirty-three storage
samples were collected with each sampler. Three of each
sampler were analyzed on the day of generation. Fifteen of
each sampler were stored at reduced temperature (about 4°C)
and the other fifteen were stored in a closed drawer at
ambient temperature (about 22°C). Three samples were selected
from each of the storage sets and analyzed at 2-4 day
intervals. Sample results were not corrected for extraction
efficiency.
Table
4.5.2.1
Storage tests for MEK on
SKC 575-002
Passive Samplers |
|
time
(days) |
|
ambient storage
recovery
(%) |
|
refrigerated
storage
recovery (%) |
|
0
2
5
8
12
15 |
|
96.6
95.5
90.2
83.6
84.1
79.3 |
98.9
95.7
90.5
86.0
84.3
79.5 |
89.2
98.5
87.2
89.9
83.0
78.8 |
|
96.6
94.6
95.9
90.5
91.2
91.3 |
98.9
97.7
96.8
92.0
91.2
90.3 |
89.2
99.5
95.8
93.8
93.2
93.7 |
|
Table
4.5.2.2
Storage tests for MIBK on
SKC 575-002
Passive Samplers |
|
time
(days) |
|
ambient storage
recovery
(%) |
|
refrigerated
storage
recovery (%) |
|
0
2
5
8
12
15 |
|
96.9
96.6
92.1
89.9
89.3
84.8 |
99.2
95.9
93.2
93.0
89.7
85.1 |
89.8
97.0
88.1
92.5
89.3
85.7 |
|
96.9
94.3
95.2
86.7
89.9
87.5 |
99.2
96.7
95.0
91.4
89.6
87.6 |
89.8
98.4
93.9
93.0
90.5
89.4 |
|
Table
4.5.2.3
Storage Tests
for MEK on 3M 3520
OVMs |
|
time
(days) |
|
ambient storage
recovery
(%) |
|
refrigerated
storage
recovery (%) |
|
0
2
5
8
12
15 |
|
96.9
96.5
98.7
89.2
94.0
99.8 |
101.3
96.6
92.9
100.5
92.8
96.2 |
92.8
89.5
92.4
92.7
100.2
98.0 |
|
96.6
96.9
101.8
92.7
104.1
98.8 |
101.3
93.5
97.6
97.6
91.5
93.9 |
92.8
94.6
97.5
92.3
95.1
102.8 |
|
Table
4.5.2.4
Storage Tests
for MIBK on 3M 3520
OVMs |
|
time
(days) |
|
ambient storage
recovery
(%) |
|
refrigerated
storage
recovery (%) |
|
0
2
5
8
12
15 |
|
101.2
101.8
103.7
95.3
99.7
105.5 |
104.9
101.2
100.1
105.8
100.9
103.3 |
100.5
94.9
98.7
99.2
105.0
103.0 |
|
101.2
102.4
109.4
97.8
108.5
103.1 |
104.9
99.4
103.3
105.3
97.3
98.7 |
100.5
100.5
102.1
96.9
101.8
106.9 |
|
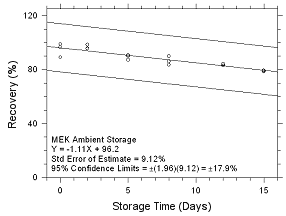
Figure
4.5.2.1 Ambient Storage for MEK collected on SKC 575-002
Passive Samplers. |
|
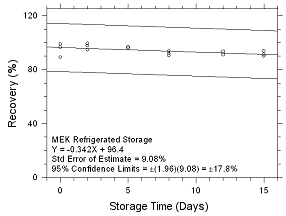
Figure
4.5.2.2 Refrigerated Storage for MEK collected on SKC 575-002
Passive Samplers. |
|

Figure
4.5.2.3 Ambient Storage for MIBK collected on SKC 575-002
Passive Samplers. |
|

Figure
4.5.2.4 Refrigerated Storage for MIBK collected on SKC 575-002
Passive Samplers. |
|
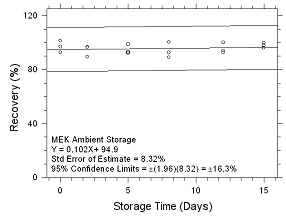
Figure
4.5.2.5 Ambient storage for MEK collected on 3M 3520 OVMs. |
|
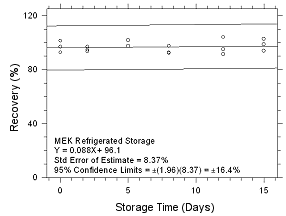
Figure
4.5.2.6 Refrigerated storage for MEK collected on 3M 3520
OVMs. |
|

Figure
4.5.2.7 Ambient storage for MIBK collected on 3M 3520 OVMs. |
|

Figure
4.5.2.8 Refrigerated storage for MIBK collected on 3M 3520
OVMs. |
4.6
Reproducibility
Six samples for each of the three
samplers evaluated in this method were collected from a
controlled test atmosphere and were submitted to OSHA SLTC for
analysis. The concentration of the test atmosphere was 593.46
mg/m3 for MEK and 393.68 mg/m3 for MIBK.
The absolute humidity was 16.9 milligrams of water per liter of
air (77.8% RH at 24°C). The face velocity of the sampled air was
0.4 m/s. The samples were analyzed utilizing a draft copy of
this procedure for analyst instruction. The samples were
analyzed after being stored for 2 days at 4°C. Sample results
were corrected for extraction efficiency. No sample result for
either analyte had a deviation greater than the precision of the
overall procedure determined in Section
4.4.
Table
4.6.1
Reproducibility Data for MEK and MIBK Collected on
SKC CMS Sampling Tubes |
|
MEK
|
|
MIBK
|
theoretical
(µg/sample) |
recovered
(µg/sample) |
recovery
(%) |
deviation
(%) |
|
theoretical
(µg/sample) |
recovered
(µg/sample) |
recovery
(%) |
deviation
(%) |
|
7157.1
6991.0
7151.2
6688.3
7353.0
6991.0 |
6715.1
6514.4
6551.4
6101.4
6743.1
6570.5 |
93.8
93.2
91.6
91.2
91.7
94.0 |
-6.2
-6.8
-8.4
-8.8
-8.3
-6.0 |
|
4747.8
4367.6
4743.8
4436.8
4877.7
4637.6 |
4508.6
4388.5
4331.7
4137.9
4379.1
4399.1 |
95.0
100.5
91.3
93.3
89.8
94.9 |
-5.0
0.5
-8.7
-6.7
-10.2
-5.1
|
|
Table 4.6.2
Reproducibility Data for MEK and
MIBK Collected on SKC 575-002 Passive Samplers |
|
MEK
|
|
MIBK
|
theoretical
(µg/sample) |
recovered
(µg/sample) |
recovery
(%) |
deviation
(%) |
|
theoretical
(µg/sample) |
recovered
(µg/sample) |
recovery
(%) |
deviation
(%) |
|
2772.7
2772.7
2772.7
2772.7
2772.7
2772.7 |
2719.3
3062.3
2818.2
2720.2
2738.9
2688.3 |
98.1
110.4
101.6
98.1
98.8
97.0 |
-1.9
10.4
1.6
-1.9
-1.2
-3.0 |
|
1484.1
1484.1
1484.1
1484.1
1484.1
1484.1 |
1548.1
1729.8
1602.4
1558.0
1598.7
1559.5 |
104.3
116.6
108.0
105.0
107.7
105.1 |
4.3
16.6
8.0
5.0
7.7
5.1 |
|
Table
4.6.3
Reproducibility Data for MEK and MIBK Collected on 3M
3520 OVMs |
|
MEK
|
|
MIBK
|
theoretical
(µg/sample) |
recovered
(µg/sample) |
recovery
(%) |
deviation
(%) |
|
theoretical
(µg/sample) |
recovered
(µg/sample) |
recovery
(%) |
deviation
(%) |
|
5353.1
5353.1
5353.1
5353.1
5353.1
5353.1 |
5430.2
5398.6
5469.8
5488.4
5112.4
4725.2 |
101.4
100.9
102.2
102.5
95.5
88.3 |
1.4
0.9
2.2
2.5
-4.5
-11.7 |
|
2942.0
2942.0
2942.0
2942.0
2942.0
2942.0 |
3194.7
3171.8
3193.9
3216.8
3102.2
2957.9 |
108.6
107.8
108.6
109.3
105.4
100.5 |
8.6
7.8
8.6
9.3
5.4
0.5 |
|
4.7 Sampler capacity
4.7.1 SKC Anasorb CMS sampling tubes
The
sampling capacity of the front section of SKC Anasorb CMS
sampling tubes was tested by sampling from a dynamically
generated test atmosphere of 1131 mg/m3 (384 ppm) MEK
and 774 mg/m3 (189 ppm) MIBK with an absolute
humidity of 13.9 milligrams of water per liter of air (74%
relative humidity at 22°C). Four samples were collected at about
50 mL/min. CMS sampling tubes were placed in-line behind the
front test sections and they were changed every 15 min after
initial collection for four hours. Five-percent breakthrough for
MEK occurred after 15 L of air had been sampled. This air volume
is that which would be collected in a 5-h air sample.
Breakthrough of MIBK was never observed in any of the sampler
capacity tests in which samples were collected for as long as
ten hours. The recommended sampling time for SKC Anasorb CMS
sampling tubes is 4 hours. This sampling time provides a
considerable capacity safety margin for MEK because of the
presence of MIBK in the test atmosphere.
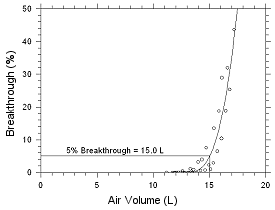
Figure 4.7.1 Five percent breakthrough
air volume for MEK from SKC Anasorb CMS
Table
4.7.1
Breakthrough of MEK from SKC Anasorb CMS |
|
| test no. |
air vol.
(L) |
sampling time
(min) |
downstream
concn
(mg/m3) |
breakthrough
(%) |
|
| 1 |
11.97
12.72
13.47
14.22
14.96
15.71
16.46
17.21
17.96 |
240
15
15
15
15
15
15
15
15 |
0.0
0.0
0.0
6.7
25.8
73.4
212.3
493.1
915.6 |
0.0
0.0
0.0
0.6
2.3
6.5
18.8
43.6
81.0 |
| |
| 2 |
11.70
12.43
13.17
13.90
14.63
15.36
16.09
16.82
17.55 |
240
15
15
15
15
15
15
15
15 |
0.0
0.0
0.0
0.0
7.7
33.3
117.9
286.4
625.8 |
0.0
0.0
0.0
0.0
0.7
2.9
10.4
25.3
55.3 |
| |
| 3 |
12.09
12.84
13.60
14.35
15.11
15.86
16.82
17.37
18.13 |
240
15
15
15
15
15
15
15
15 |
0.0
0.0
9.2
44.3
11.5
211.5
360.6
613.2
879.8 |
0.0
0.0
0.8
3.9
1.0
18.7
31.9
54.2
77.8 |
| |
| 4 |
11.21
11.91
12.61
13.31
14.01
14.71
15.41
16.11
16.81 |
240
15
15
15
15
15
15
15
15 |
0.0
0.0
4.2
12.0
35.7
84.9
153.1
326.8
598.4 |
0.0
0.0
0.4
1.1
3.2
7.5
13.5
28.9
52.9 |
|
4.7.2
Diffusive samplers
Sampling rate and sampler capacity
were determined using SKC and 3M samplers that were exposed to a
controlled test atmosphere for increasing time intervals. Three
of each sampler were exposed for each time interval. Sampler
capacity is defined as exceeded when the sampling rate appears
to decrease, as was the case for MEK collected on 3M 3520 OVMs.
The concentration of the test atmospheres was about two times
the target concentration with an absolute humidity of about 16.4
milligrams of water per liter of air (80% RH at 23°C).
Preliminary sampling rates were determined by averaging the
values for the 0.5, 1 and 2 h samples. Horizontal lines were
placed 10% above and 10% below the preliminary sampling rates.
Sampling rates were calculated by averaging all the individual
sampling rates that were between the two horizontal lines.
Sampling rates, RSDs of individual runs, and RSDs of average
sampling rates are shown in Table 4.7.2.1. Data shown in Table
4.7.2.1 are for the front section of the 3M samplers. A
five-hour experiment was performed for the 3M sampler because
its capacity for MEK was exceeded before six hours. The
recommended sampling time is four hours.
Table
4.7.2.1
Determination of Sampling Rate and Recommneded
Sampling Time |
|
| |
SKC 575-002 Passive Sampler
|
|
3M 3520 OVM
|
| |
MEK |
MIBK |
|
MEK |
MIBK |
| time(h) |
(mL/min) |
RSD |
(mL/min) |
RSD(%) |
|
(mL/min) |
RSD |
(mL/min) |
RSD(%) |
|
0.083
0.167
0.50
1.00
2.00
3.00
4.00
5.00
6.00
8.00
10.00 |
16.12
16.87
16.55
17.38
17.53
17.21
16.87
16.88
16.94
16.49 |
6.4
3.2
0.5
1.8
0.6
0.9
3.8
0.5
1.8
1.5 |
14.32
13.58
13.84
13.47
13.59
13.60
13.52
13.29
13.62
13.40 |
3.2
1.7
2.8
1.3
0.7
0.8
3.8
0.5
3.4
1.5 |
|
33.28
33.80
33.52
33.64
31.21
32.22
31.36
31.71
27.24
22.47
17.83 |
0.3
3.9
1.6
3.3
1.5
4.4
0.8
3.8
5.0
3.5
2.2 |
27.29
27.39
26.64
26.95
25.74
26.88
26.49
27.50
26.64
28.04
27.41 |
3.9
3.6
0.7
1.4
0.9
4.3
3.8
3.8
3.4
1.6
2.2 |
| |
| mean |
16.88 |
|
13.62 |
|
|
32.59 |
|
27.00 |
|
| RSD(%) |
2.5 |
|
2.1 |
|
|
3.3 |
|
2.3 |
|
|

Figure
4.7.2.1 Sampler capcity Data for MEK collected on SKC 575-002
Passive Sampler. |
|
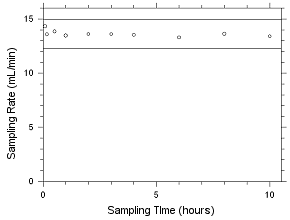
Figure
4.7.2.2 Sampler capcity Data for MIBK collected on SKC 575-002
Passive Sampler. |
| |

Figure
4.7.2.3 Sampler capcity Data for MEK collected on 3M 3520
OVM. |
|

Figure
4.7.2.4 Sampler capcity Data for MIBK collected on 3M 3520
OVM. |
Sampling rate is normally
calculated using only the front section of two-section
samplers. The 3M 3520 OVM is a two-section sampler. When MEK
collected on the back section was included in the total
amount, the resultant MEK sampling rates were comparable with
those obtained above. This data should not be used to justify
sampling times longer than four hours, but it does show the
value of a two-section sampler.
Table
4.7.2.2
MEK Sampling Rates for
3M 3520 OVM With
Back Section Included |
|
| time (h) |
mL/min |
RSD (%) |
|
6
8
10 |
31.54
32.84
32.29 |
4.0
0.7
1.5 |
| 4.8 Extraction efficiency and stability of
extracted samples
A summary of extraction efficiencies
(at concentrations near the RQL to 2 times the target
concentration) for all the sampling media used in this work is
shown in Table 4.8. The extraction efficiency is dependent on
the extraction solvent as well as the internal standard. Other
extraction solvents or internal standards may be used provided
they are tested as described below.
Table
4.8
Extraction Efficiency Summary |
|
| medium |
MEK (%) |
MIBK (%) |
|
Anasorb CMS
Anasorb 747
SKC
575-002
3M 3520 OVM |
100.3
97.9
92.2
98.0 |
102.3
99.0
92.9
96.5 |
|
4.8.1 SKC Anasorb CMS sampling
tubes
Extraction efficiency
The extraction
efficiencies of MEK and MIBK were determined by liquid-spiking
150 mg portions of Anasorb CMS with the analytes at
concentrations from the RQL to 2 times the target
concentration. These samplers were stored overnight at ambient
temperature and then analyzed. The mean extraction efficiency
over the working range of the RQL to 2 times the target
concentration is 100.3% for MEK and 102.3% for MIBK. The
extraction efficiency for MEK from wet samplers was lower than
for dry samplers. These results were expected because of the
instability of MEK on wet carbon-based sorbents. The
extraction efficiency for the wet samplers was not included in
the overall mean because it would bias the results. Wet
sampling media used in extraction efficiency tests were all
prepared by sampling contaminant-free, humid air (about 80%
relative humidity at 22°C) for four hours at the recommended
sampling rate.
Table
4.8.1.1
Extraction Efficiency of MEK from Anasorb CMS
(%) |
|
level
|
sample number
|
|
× target
concn |
µg per
sample |
1 |
2 |
3 |
4 |
mean |
|
RQL
0.25
0.5
1.0
1.5
2.0
1.0
(wet) |
0.808
1781
3562
7125
10450
14250
7125 |
100.7
98.4
99.7
104.0
101.8
101.3
89.9 |
101.6
98.1
101.0
96.9
101.1
100.6
86.3 |
92.8
98.4
98.2
104.7
101.8
99.5
87.1 |
98.7
98.7
100.6
104.4
99.6
103.5
91.5 |
98.5
98.4
99.9
102.5
101.1
101.2
88.7 |
|
Table
4.8.1.2
Extraction Efficiency of MIBK from Anasorb
CMS (%) |
|
level
|
sample number
|
|
× target
concn |
µg per
sample |
1 |
2 |
3 |
4 |
mean |
|
RQL
0.25
0.5
1.0
1.5
2.0
1.0
(wet) |
0.423
1219
2438
4876
7152
9753
4876 |
111.0
98.6
98.0
103.7
101.4
100.8
98.6 |
111.9
98.7
99.8
98.0
102.3
100.4
95.4 |
114.5
98.7
97.8
104.1
101.8
99.6
96.2 |
109.3
98.4
99.1
103.6
100.4
102.7
99.5 |
111.7
98.6
98.7
102.4
101.5
100.9
97.4 |
|
Stability of extracted samples
The
stability of extracted samples was investigated by reanalyzing
the target concentration samples a day after the initial
analysis. After the original analysis was performed two vials
were recapped with new septa while the remaining two retained
their punctured septa. The samples were reanalyzed with fresh
standards. Each septum was punctured 6 times for each
injection.
Table
4.8.1.3
Stability of Samples
for MEK Extracted
From Anasorb CMS |
|
punctured septa replaced
|
|
punctured septa retained
|
initial
(%) |
after one day
(%) |
difference
(%) |
|
initial
(%) |
after one day
(%) |
difference
(%) |
|
104.0
96.9
100.5 |
100.3
100.2
mean
100.3 |
-3.7
3.3
-0.2 |
|
104.7
104.4
104.6 |
89.3
84.9
mean
87.1 |
-15.4
-19.5
-17.5 |
|
Table
4.8.1.4
Stability of Samples
for MIBK Extracted
From Anasorb CMS |
|
punctured septa replaced
|
|
punctured septa retained
|
initial
(%) |
after one day
(%) |
difference
(%) |
|
initial
(%) |
after one day
(%) |
difference
(%) |
|
103.7
98.0
100.9 |
100.8
100.2
mean
100.5 |
-2.9
2.2
-0.4 |
|
104.1
103.6
103.9 |
96.8
96.8
mean
96.8 |
-7.3
-6.8
-7.1 |
|
The
stability test for MEK was repeated with the reanalysis
performed 12 hours after the initial analysis. After the
original analysis was performed, two vials were recapped with
new septa while the remaining two retained their punctured
septa. The samples were reanalyzed with fresh standards. Each
septum was punctured 6 times for each injection.
Table
4.8.1.5
Stability of Samples
for MEK Extracted
From Anasorb CMS |
|
punctured septa replaced
|
|
punctured septa retained
|
initial
(%) |
after 12 h
(%) |
difference
(%) |
|
initial
(%) |
after 12 h
(%) |
difference
(%) |
|
98.3
97.5
97.9 |
96.6
96.1
mean
96.4 |
-1.7
-1.4
-1.6 |
|
99.1
99.0
99.1 |
92.6
94.0
mean
93.3 |
-6.5
-5.0
-5.8 |
|
4.8.2
SKC Anasorb 747 sampling tubes
Extraction efficiency
The extraction efficiencies of MEK and MIBK were
determined by liquid-spiking 400 mg portions of Anasorb 747
with the analytes at concentrations from the RQL to 2 times
the target concentration. These samplers were stored overnight
at ambient temperature and then analyzed. The mean extraction
efficiency over the working range of the RQL to 2 times the
target concentration is 97.9% for MEK and 99.0% for MIBK. The
extraction efficiency for MEK from wet samplers was lower than
for dry samplers. These results were expected because of the
instability of MEK on wet sorbents. The extraction efficiency
for the wet samplers was not included in the overall mean
because it would bias the results.
Table
4.8.2.1
Extraction Efficiency of MEK from Anasorb 747
(%) |
|
level
|
sample number
|
|
× target
concn |
µg per
sample |
1 |
2 |
3 |
4 |
mean |
|
RQL
0.25
0.5
1.0
1.5
2.0
1.0
(wet) |
0.950
1781
3562
7125
10450
14250
7125 |
86.5
94.4
94.8
98.8
101.9
92.7
87.3 |
114.5
94.9
97.1
98.8
99.7
98.7
86.4 |
104.1
94.5
94.7
99.4
99.1
99.0
86.0 |
100.7
94.8
95.4
96.7
99.5
98.2
91.7 |
101.5
94.7
95.5
98.4
100.1
97.2
87.9 |
|
Table
4.8.2.2
Extraction Efficiency of MIBK from Anasorb
747 (%) |
|
level
|
sample number
|
|
× target
concn |
µg per
sample |
1 |
2 |
3 |
4 |
mean |
|
RQL
0.25
0.5
1.0
1.5
2.0
1.0
(wet) |
0.683
1219
2438
4876
7152
9753
4876 |
103.6
97.3
96.9
100.2
102.9
96.0
96.3 |
91.1
96.6
99.6
101.1
101.3
100.7
95.0 |
100.2
96.6
96.8
101.7
101.0
100.7
94.9 |
97.8
96.6
97.4
99.5
101.3
100.2
99.6 |
98.2
96.8
97.7
100.6
101.6
99.4
96.5 |
|
Stability of extracted samples
The
stability of extracted samples was investigated by reanalyzing
the target concentration samples a day after initial analysis.
After the original analysis was performed, two vials were
recapped with new septa while the remaining two retained their
punctured septa. Each septum was punctured 6 times for each
injection.
Table
4.8.2.3
Stability of Samples
for MEK Extracted
From Anasorb 747 |
|
punctured septa replaced
|
|
punctured septa retained
|
initial
(%) |
after one day
(%) |
difference
(%) |
|
initial
(%) |
after one day
(%) |
difference
(%) |
|
98.8
98.8
98.8 |
97.8
100.2
mean
99.0 |
-1.0
1.4
0.2 |
|
99.4
96.7
98.1 |
88.6
84.4
mean
86.5 |
-10.8
-12.3
-11.6 |
|
Table
4.8.2.4
Stability of Samples
for MIBK Extracted
From Anasorb 747 |
|
punctured septa replaced
|
|
punctured septa retained
|
initial
(%) |
after one day
(%) |
difference
(%) |
|
initial
(%) |
after one day
(%) |
difference
(%) |
|
100.2
101.1
100.7 |
100.5
102.1
mean
101.3 |
0.3
1.0
0.7 |
|
101.7
99.5
100.6 |
98.0
96.0
mean
97.0 |
-3.7
-3.5
-3.6 |
|
The
stability test for MEK was repeated with the reanalysis
performed 12 hours after the initial analysis. After the
original analysis was performed, two vials were recapped with
new septa while the remaining two retained their punctured
septa. The samples were reanalyzed with fresh standards. Each
septum was punctured 6 times for each injection.
Table
4.8.2.5
Stability of Samples
for MEK Extracted
From Anasorb 747 |
|
punctured septa replaced
|
|
punctured septa retained
|
initial
(%) |
after 12 h
(%) |
difference
(%) |
|
initial
(%) |
after 12 h
(%) |
difference
(%) |
|
98.5
97.4
98.0 |
99.0
97.4
mean
98.2 |
0.5
0.0
0.3 |
|
98.9
98.1
98.5 |
96.0
93.3
mean
94.7 |
-2.9
-4.8
-3.9 |
|
4.8.3
SKC 575-002 Passive Samplers
Extraction efficiency
The extraction efficiencies of MEK and MIBK were
determined by liquid spiking SKC 575-002 Passive Samplers with
the analytes at concentrations from the RQL to 2 times the
target concentration. These samplers were stored overnight at
ambient temperature and then analyzed. The mean extraction
efficiency over the working range of the RQL to 2 times the
target concentration is 92.2% for MEK and 92.9% for MIBK. The
extraction efficiency for MEK from wet samplers was lower than
for dry samplers. These results were expected because of the
instability of MEK on wet sorbents. The extraction efficiency
for the wet samplers was not included in the overall mean
because it would bias the results.
Table
4.8.3.1
Extraction Efficiency of MEK
from SKC
575-002 Passive Samplers (%) |
|
level
|
sample number
|
|
× target
concn |
µg per
sample |
1 |
2 |
3 |
4 |
mean |
|
RQL
0.25
0.5
1.0
1.5
2.0
1.0
(wet) |
1.282
594
1188
2375
3800
4750
2375 |
80.8
92.7
87.4
94.4
89.9
95.1
89.8
|
101.8
97.2
87.6
92.4
94.1
93.4
85.7
|
85.5
91.8
90.9
99.4
90.2
91.5
83.7
|
93.3
95.1
89.3
94.7
95.2
88.5
87.9
|
90.4
94.2
88.8
95.2
92.4
92.1
86.8 |
|
Table
4.8.3.2
Extraction Efficiency of MIBK
from SKC
575-002 Passive Samplers (%) |
|
level
|
sample number
|
|
× target
concn |
µg per
sample |
1 |
2 |
3 |
4 |
mean |
|
RQL
0.25
0.5
1.0
1.5
2.0
1.0
(wet) |
1.268
325
650
1300
1950
2601
1300 |
71.6
91.0
94.8
98.2
98.1
98.2
97.0 |
74.7
92.9
96.6
97.2
97.6
92.1
96.6 |
94.3
90.0
92.8
99.4
97.0
95.3
97.7 |
83.3
90.9
94.9
96.0
95.5
97.5
99.5 |
81.0
91.2
94.8
97.7
97.1
95.8
97.7 |
|
Stability of extracted samples
The
stability of extracted samples was investigated by reanalyzing
the target concentration samples a day after initial analysis.
After the original analysis was performed, two vials were
recapped with new septa while the remaining two retained their
punctured septa. The samples were reanalyzed with fresh
standards. Each septum was punctured 6 times for each
injection.
Table
4.8.3.3
Stability of Samples for MEK
Extracted
From SKC 575-002 Passive Samplers |
|
punctured septa replaced
|
|
punctured septa retained
|
initial
(%) |
after one day
(%) |
difference
(%) |
|
initial
(%) |
after one day
(%) |
difference
(%) |
|
94.4
92.4
93.4 |
93.0
92.7
mean
92.9 |
-1.4
0.3
-0.6 |
|
99.4
94.7
97.1 |
77.2
83.6
mean
80.4 |
-22.2
-11.1
-16.7 |
|
Table
4.8.3.4
Stability of Samples for MIBK
Extracted
From SKC 575-002 Passive Samplers |
|
punctured septa replaced
|
|
punctured septa retained
|
initial
(%) |
after one day
(%) |
difference
(%) |
|
initial
(%) |
after one day
(%) |
difference
(%) |
|
98.2
97.2
97.7 |
95.3
97.0
mean
96.2 |
-2.9
-0.2
-1.6 |
|
99.4
96.0
97.7 |
92.8
87.8
mean
90.3 |
-6.6
-8.2
-7.4 |
|
The
stability test for MEK was repeated with the reanalysis
performed 12 hours after the initial analysis. After the
original analysis was performed, two vials were recapped with
new septa while the remaining two retained their punctured
septa. The samples were reanalyzed with fresh standards. Each
septum was punctured 6 times for each injection.
Table
4.8.3.5
Stability of Samples for MEK
Extracted
From SKC 575-002 Passive Samplers |
|
punctured septa replaced
|
|
punctured septa retained
|
initial
(%) |
after 12 h
(%) |
difference
(%) |
|
initial
(%) |
after 12 h
(%) |
difference
(%) |
|
89.7
90.3
90.0 |
94.6
93.2
mean
93.9 |
4.9
2.9
3.9 |
|
89.7
94.5
92.1 |
89.6
86.4
mean
88.0 |
-0.1
-8.1
-4.1 |
|
4.8.4 3M
3520 OVM
Extraction efficiency
The extraction
efficiencies of MEK and MIBK were determined by liquid-spiking
3M 3520 OVM charcoal wafers with the analytes at
concentrations from the RQL to 2 times the target
concentration. These samples were stored overnight at ambient
temperature and then analyzed. The mean extraction efficiency
over the working range of the RQL to 2 times the target
concentration is 98.0% for MEK and 96.5% for MIBK. The
extraction efficiency for MEK from wet samplers was lower than
for dry samplers. These results were expected because of the
instability of MEK on wet sorbents. The extraction efficiency
for the wet samplers was not included in the overall mean
because it would bias the results.
Table
4.8.4.1
Extraction Efficiency of MEK
from 3M 3520
OVM Charcoal Wafers (%) |
|
level
|
sample number
|
|
× target
concn |
µg per
sample |
1 |
2 |
3 |
4 |
mean |
|
RQL
0.25
0.5
1.0
1.5
2.0
1.0
(wet) |
0.760
1188
2375
4750
7125
9500
4750 |
108.6
100.1
97.9
96.4
97.1
89.4
88.2 |
91.7
93.2
102.1
96.8
95.6
98.8
89.3 |
107.2
92.4
103.8
94.0
100.9
95.7
90.1 |
113.7
94.7
98.3
95.0
93.9
94.3
91.4 |
105.3
95.1
100.5
95.6
96.9
94.6
89.8 |
|
Table
4.8.4.2
Extraction Efficiency of MIBK
from 3M 3520
OVM Charcoal Wafers (%) |
|
level
|
sample number
|
|
× target
concn |
µg per
sample |
1 |
2 |
3 |
4 |
mean |
|
RQL
0.25
0.5
1.0
1.5
2.0
1.0
(wet) |
0.943
650
1300
2601
3901
5201
2601 |
92.0
98.2
97.2
99.7
98.5
100.7
98.7 |
87.3
96.1
98.7
98.2
101.3
97.2
97.5 |
84.8
93.2
98.4
96.8
98.3
103.0
93.8 |
81.5
97.1
98.9
96.2
100.2
101.3
98.1 |
86.4
96.2
98.3
97.7
99.6
100.6
97.0 |
|
Stability of extracted samples
The
stability of extracted samples was investigated by reanalyzing
the target concentration samples a day after initial analysis.
After the original analysis was performed, two vials were
recapped with new septa while the remaining two retained their
punctured septa. The samples were reanalyzed with fresh
standards. Each septum was punctured 6 times for each
injection.
Table
4.8.4.3
Stability of Samples for MEK
Extracted
From 3M 3520 OVM Charcoal Wafers |
|
punctured septa replaced
|
|
punctured septa retained
|
initial
(%) |
after one day
(%) |
difference
(%) |
|
initial
(%) |
after one day
(%) |
difference
(%) |
|
96.4
96.8
96.6 |
97.2
94.3
mean
95.8 |
0.8
-2.5
-0.9 |
|
94.0
95.0
94.5 |
84.2
77.9
mean
81.1 |
-9.8
-17.1
-13.5 |
|
Table
4.8.4.4
Stability of Samples for MIBK
Extracted
From 3M 3520 OVM Charcoal Wafers |
|
punctured septa replaced
|
|
punctured septa retained
|
initial
(%) |
after one day
(%) |
difference
(%) |
|
initial
(%) |
after one day
(%) |
difference
(%) |
|
99.7
98.2
99.0 |
99.4
100.1
mean
99.8 |
-0.3
1.9
0.8 |
|
96.8
96.2
96.5 |
93.8
96.1
mean
95.0 |
-3.0
-0.1
-1.6 |
|
The
stability test for MEK was repeated with the reanalysis
performed 12 hours after the initial analysis. After the
original analysis was performed, two vials were recapped with
new septa while the remaining two retained their punctured
septa. The samples were reanalyzed with fresh standards. Each
septum was punctured 6 times for each injection.
Table
4.8.4.5
Stability of Samples for MEK
Extracted
From 3M 3520 OVM Charcoal Wafers |
|
punctured septa replaced
|
|
punctured septa retained
|
initial
(%) |
after 12 h
(%) |
difference
(%) |
|
initial
(%) |
after 12 h
(%) |
difference
(%) |
|
100.0
103.5
101.8 |
95.3
94.9
mean
95.1 |
-4.7
-8.6
-6.7 |
|
102.7
103.6
103.2 |
94.1
94.4
mean
94.3 |
-8.6
-9.2
-8.9 |
|
4.9 Interferences (sampling)
4.9.1 SKC Anasorb CMS sampling tubes
Retention
The ability of SKC Anasorb CMS
sampling tubes to retain MEK and MIBK after collection was
tested by sampling an atmosphere containing 1178
mg/m3 MEK and 806 mg/m3 MIBK at an
absolute humidity of 14.6 milligrams of water per liter of air
(77.8% relative humidity at 21.4°C). Six samplers had
contaminated air drawn through them at about 50 mL/min for 60
min. Sampling was discontinued and three samples set aside.
The generation system was flushed with contaminant-free air.
Sampling was resumed with the other three samples with
contaminant-free humid air drawn through them at 50 mL/min for
3 h and then all six samplers were analyzed. Recoveries for
the two sets are expressed as percent of theoretical.
Table
4.9.1
Retention (%) of MEK and MIBK on Anasorb
CMS |
|
| |
1 |
2 |
3 |
mean |
| set |
MEK |
MIBK |
MEK |
MIBK |
MEK |
MIBK |
MEK |
MIBK |
|
first
second
second/first |
96.7
92.2
|
96.1
93.4
|
96.2
93.1
|
94.7
93.4
|
97.2
91.9
|
96.5
92.5
|
96.7
92.4
95.6 |
95.8
93.1
97.2 |
|
Low
humidity
The ability of SKC Anasorb CMS sampling tubes
to collect MEK and MIBK from a relatively dry atmosphere was
tested by sampling an atmosphere containing 1203
mg/m3 of MEK and 823 mg/m3 of MIBK at an
absolute humidity of 2.7 milligrams of water per liter of air
(13.2% relative humidity at 22.6°C). Three samplers had
contaminated air drawn through them at 50 mL/min for 240 min.
All three samples were immediately analyzed. The sample
results were 96.3%, 97.2% and 95.1% of theoretical for MEK;
and 98.6%, 99.3%, and 98.2% of theoretical for MIBK.
Low concentration
The ability of SKC Anasorb
CMS sampling tubes to collect MEK and MIBK at low
concentrations was tested by sampling an atmosphere containing
60.4 mg/m3 of MEK and 41.3 mg/m3 of MIBK
at an absolute humidity of 15.5 milligrams of water per liter
of air (79.7% relative humidity at 22.3°C). Three samplers had
contaminated air drawn through them at 50 mL/min for 240 min.
All three samples were immediately analyzed. The sample
results were 94.9%, 98.2% and 97.2% of theoretical for MEK and
101.1%, 104.7% and 104.4% of theoretical for MIBK.
Sampling interferences
The ability of SKC
Anasorb CMS sampling tubes to collect MEK and MIBK was tested
in the presence of potential interferences by sampling an
atmosphere containing 578 mg/m3 of MEK and 395
mg/m3 of MIBK at an absolute humidity of 15.6
milligrams of water per liter of air (79.0% relative humidity
at 22.23°C) and 553.3 mg/m3 acetone, 253.7
mg/m3 isopropyl alcohol, 190.1 mg/m3
toluene, 92.5 mg/m3 xylene isomers, and 16.3
mg/m3 ethyl benzene. Three samplers had
contaminated air drawn through them at 50 mL/min for 240 min.
All three samples were immediately analyzed. The sample
results were 99.1%, 99.3% and 99.0% of theoretical for MEK;
and 102.2%, 103.0% and 102.4% of theoretical for MIBK.
4.9.2 SKC 575-002 Passive Samplers and 3M 3520 OVMs
Reverse diffusion
The ability of SKC 575-002
Passive Samplers and of 3M 3520 OVMs to retain MEK and MIBK
after collection was tested by sampling an atmosphere
containing 1178 mg/m3 MEK and 806 mg/m3
MIBK at an absolute humidity of 14.6 milligrams of water per
liter of air (77.8% relative humidity at 21.4°C). Six of each
sampler were exposed to contaminated air for one hour.
Sampling was discontinued and three of each sampler were set
aside (1st set). The generation system was flushed
with contaminant-free air. Sampling was resumed with the
remaining six samplers exposed to contaminant-free humid air
for an additional three hours (2nd set). Results
are presented in terms of micrograms of analyte found on the
sampler. The means of the second set were 99.3% for MEK and
100.0% for MIBK of the means of the first set for SKC 575-002
Passive Samplers. The means of the second set were 96.0% for
MEK and 98.6% for MIBK of the means of the first set for 3M
3520 OVMs.
Table
4.9.2.1
Reverse Diffusion For SKC 575-002 Passive
Sampler (µg) |
|
| set |
1 |
2 |
3 |
|
| |
MEK |
MIBK |
MEK |
MIBK |
MEK |
MIBK |
first
second |
1357.45
1355.55 |
741.52
739.86 |
1368.54
1360.50 |
732.50
737.37 |
1405.26
1387.31 |
753.41
750.30 |
|
means |
first
second |
1377.08
1367.79 |
742.48
742.51 |
|
Table
4.9.2.2
Reverse Diffusion For 3M OVM (µg) |
|
| set |
1 |
2 |
3 |
|
| |
MEK |
MIBK |
MEK |
MIBK |
MEK |
MIBK |
first
second |
2700.73
2580.20 |
1520.43
1471.29 |
2627.96
2607.50 |
1464.63
1510.12 |
2811.44
2628.50 |
1535.59
1477.16 |
| means
|
first
second |
2713.38
2605.40 |
1506.88
1486.19 |
|
Low
humidity
Three SKC and three 3M diffusive samplers
were used to sample a test atmosphere containing 1203
mg/m3 MEK and 823 mg/m3 MIBK. The
absolute humidity of the test atmosphere was 2.7 milligrams of
water per liter of air (13.2% relative humidity at 22.6°C).
The recoveries (% theoretical) are shown in Table 4.9.2.3.
Table
4.9.2.3
Low Humidity
(Recovery, % of
Theoretical) |
|
| sampler |
1 |
|
2 |
|
3 |
| |
MEK |
MIBK |
|
MEK |
MIBK |
|
MEK |
MIBK |
|
SKC 575-002
3M 3520 |
|
92.2
95.5 |
102.5
109.2 |
|
94.9
93.9 |
104.9
102.6 |
|
92.8
92.9 |
103.1
101.0 |
|
Low
concentration
Three SKC and three 3M diffusive
samplers were used to sample a test atmosphere containing 60.3
mg/m3 MEK and 41.3 mg/m3 MIBK. The
absolute humidity of the test atmosphere was 15.5 milligrams
of water per liter of air (79.7% relative humidity at 22.3°C).
The recoveries (% theoretical) are shown in Table 4.9.2.4.
Table
4.9.2.4
Low Concentration
(Recovery, % of
Theoretical) |
|
| sampler |
1 |
|
2 |
|
3 |
| |
MEK |
MIBK |
|
MEK |
MIBK |
|
MEK |
MIBK |
|
SKC 575-002
3M 3520 |
|
92.2
89.8 |
101.6
95.0 |
|
95.6
93.4 |
105.2
97.9 |
|
90.3
92.2 |
96.8
98.5 |
|
Sampling
interferences
Three SKC and three 3M diffusive
samplers were used to sample a test atmosphere containing 578
mg/m3 of MEK and 395 mg/m3 of MIBK; and
553.3 mg/m3 of acetone, 253.7 mg/m3 of
isopropyl alcohol, 190.1 mg/m3 of toluene, 92.5
mg/m3 of xylene isomers, and 16.3 mg/m3
of ethyl benzene. The absolute humidity was 15.6 milligrams of
water per liter of air (79.0% relative humidity at 22.3°C).
The recoveries (% theoretical) are shown in Table
4.9.2.5.
Table
4.9.2.5
Sampling Interference
(Recovery, % of
Theoretical) |
|
| sampler |
1 |
|
2 |
|
3 |
| |
MEK |
MIBK |
|
MEK |
MIBK |
|
MEK |
MIBK |
|
SKC 575-002
3M 3520 |
|
103.2
95.0 |
108.5
103.1 |
|
97.6
93.6 |
102.2
101.8 |
|
101.6
93.5 |
107.3
102.8 |
|
4.10 Qualitative analysis
The identity of
suspected MEK or MIBK GC peaks can be confirmed by GC/Mass
Spectrometry. Mass spectra for the analytes are shown below.
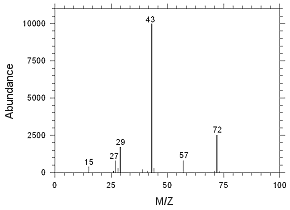
Figure
4.10.1 Mass spectrum of MEK. |
|
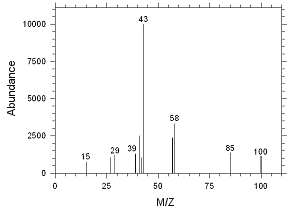
Figure
4.10.2 Mass spectrum of MIBK |
References
1. OSHA Salt Lake
Technical Center, Chemical Sampling Information, http://www.osha-slc.gov/dts/chemicalsampling/data/CH_222300.html
(accessed Feb 2000).
(Back to text)
2. OSHA
Salt Lake Technical Center, Chemical Sampling Information, http://www.osha-slc.gov/dts/chemicalsampling/data/CH_245600.html
(accessed Feb 2000).
(Back to text)
3.
Documentation of the Threshold Limit Values and
Biological Exposure Indices, 6th ed., American
Conference of Governmental Industrial Hygienists, Inc.: Cincinnati,
OH, 1991, vol. II, pp.1002-1004 and 1019-1021.
(Back to text)
4.
Supplements (1996) to the 6th Edition of: Documentation of the Threshold Limit Values and
Biological Exposure Indices, 6th ed., American Conference
of Governmental Industrial Hygienists, Inc.: Cincinnati, OH,
1991, vol. II, pp. Supplement: Methyl Isobutyl Ketone (MIBK)- BEI
1-3.
(Back to text)
5.
Documentation of the Threshold Limit Values and
Biological Exposure Indices, 6th ed., American
Conference of Governmental Industrial Hygienists, Inc.: Cincinnati,
OH, 1991, vol. II, pp.1002-1004 and 1019-1021.
(Back to text)
6.
Documentation of the Threshold Limit Values and
Biological Exposure Indices, 6th ed., American
Conference of Governmental Industrial Hygienists, Inc.: Cincinnati,
OH, 1991, vol. II, pp.1002-1004 and 1019-1021.
(Back to text)
7. OSHA
Salt Lake Technical Center, Chemical Sampling Information, http://www.osha-slc.gov/dts/chemicalsampling/toc/toc_chemsamp.html
(Accessed Jan 2000).
(Back to text)
8.Burright, D.; Chan, Y.; Eide, M.; Elskamp, C.;
Hendricks, W.; Rose, M. C. Evaluation Guidelines
for Air Sampling Methods Utilizing Chromatographic Analysis;
OSHA Salt Lake Technical Center, U.S. Department of Labor: Salt Lake
City, UT, 1999.
(Back to text)
9.Burright, D.; Chan, Y.; Eide, M.; Elskamp, C.;
Hendricks, W.; Rose, M. C. Evaluation Guidelines
for Air Sampling Methods Utilizing Chromatographic Analysis;
OSHA Salt Lake Technical Center, U.S. Department of Labor: Salt Lake
City, UT, 1999.
(Back to text)
10.
Occupational Exposure to Hazardous Chemicals in Laboratories.
Code of Federal Regulations, Part
1910.1450, Title 29, 1998.
(Back to text)
11.
Burright, D.; Chan, Y.; Eide, M.; Elskamp, C.; Hendricks, W.;
Rose, M. C. Evaluation Guidelines for Air
Sampling Methods Utilizing Chromatographic Analysis; OSHA
Salt Lake Technical Center, U.S. Department of Labor: Salt Lake
City, UT, 1999.
(Back to text)
12.
Burright, D.; Chan, Y.; Eide, M.; Elskamp, C.; Hendricks, W.;
Rose, M. C. Evaluation Guidelines for Air
Sampling Methods Utilizing Chromatographic Analysis; OSHA
Salt Lake Technical Center, U.S. Department of Labor: Salt Lake
City, UT, 1999.
(Back to text)
|

